Accuracy of the Patient Health Questionnaire-9 for screening to detect major depression: updated systematic review and individual participant data meta-analysis
- PMID: 34610915
- PMCID: PMC8491108
- DOI: 10.1136/bmj.n2183
Accuracy of the Patient Health Questionnaire-9 for screening to detect major depression: updated systematic review and individual participant data meta-analysis
Abstract
Objective: To update a previous individual participant data meta-analysis and determine the accuracy of the Patient Health Questionnaire-9 (PHQ-9), the most commonly used depression screening tool in general practice, for detecting major depression overall and by study or participant subgroups.
Design: Systematic review and individual participant data meta-analysis.
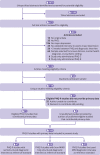
Data sources: Medline, Medline In-Process, and Other Non-Indexed Citations via Ovid, PsycINFO, Web of Science searched through 9 May 2018.
Review methods: Eligible studies administered the PHQ-9 and classified current major depression status using a validated semistructured diagnostic interview (designed for clinician administration), fully structured interview (designed for lay administration), or the Mini International Neuropsychiatric Interview (MINI; a brief interview designed for lay administration). A bivariate random effects meta-analytic model was used to obtain point and interval estimates of pooled PHQ-9 sensitivity and specificity at cut-off values 5-15, separately, among studies that used semistructured diagnostic interviews (eg, Structured Clinical Interview for Diagnostic and Statistical Manual), fully structured interviews (eg, Composite International Diagnostic Interview), and the MINI. Meta-regression was used to investigate whether PHQ-9 accuracy correlated with reference standard categories and participant characteristics.
Results: Data from 44 503 total participants (27 146 additional from the update) were obtained from 100 of 127 eligible studies (42 additional studies; 79% eligible studies; 86% eligible participants). Among studies with a semistructured interview reference standard, pooled PHQ-9 sensitivity and specificity (95% confidence interval) at the standard cut-off value of ≥10, which maximised combined sensitivity and specificity, were 0.85 (0.79 to 0.89) and 0.85 (0.82 to 0.87), respectively. Specificity was similar across reference standards, but sensitivity in studies with semistructured interviews was 7-24% (median 21%) higher than with fully structured reference standards and 2-14% (median 11%) higher than with the MINI across cut-off values. Across reference standards and cut-off values, specificity was 0-10% (median 3%) higher for men and 0-12 (median 5%) higher for people aged 60 or older.
Conclusions: Researchers and clinicians could use results to determine outcomes, such as total number of positive screens and false positive screens, at different PHQ-9 cut-off values for different clinical settings using the knowledge translation tool at www.depressionscreening100.com/phq.
Study registration: PROSPERO CRD42014010673.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Canadian Institutes of Health Research for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years with the following exceptions: Dr Bernstein has consulted to Abbvie Canada, Amgen Canada, Bristol Myers Squibb Canada, Roche Canada, Janssen Canada, Pfizer Canada, Sandoz Canada, Takeda Canada, and Mylan Pharmaceuticals; received unrestricted educational grants from Abbvie Canada, Janssen Canada, Pfizer Canada, and Takeda Canada; as well as been on speaker's bureau of Abbvie Canada, Janssen Canada, Takeda Canada and Medtronic Canada, all outside the submitted work. Dr Chan J CN is a steering committee member and/or consultant of Astra Zeneca, Bayer, Lilly, MSD and Pfizer. She has received sponsorships and honorarium for giving lectures and providing consultancy and her affiliated institution has received research grants from these companies. Dr Chan LF declares personal fees and non-financial support from Otsuka, Lundbeck, and Johnson and Johnson; and non-financial support from Ortho-McNeil-Janssen, and Menarini, outside the submitted work. Dr Hegerl declares that within the last three years, he was an advisory board member for Lundbeck and Servier; a consultant for Bayer Pharma; a speaker for Pharma and Servier; and received personal fees from Janssen Janssen and a research grant from Medice, all outside the submitted work. Dr Inagaki declares that he has received personal fees from Meiji, Mochida, Takeda, Novartis, Yoshitomi, Pfizer, Eisai, Otsuka, MSD, Sumitomo Dainippon, Janssen, and Eli Lilly, all outside of the submitted work. Dr Pugh declares that she received salary support from Pfizer-Astella and Millennium, outside the submitted work. Dr Rancans declares that he received grants, personal fees and non-financial support from Gedeon Richter; personal fees and non-financial support from Lundbeck, Servier, and Janssen Cilag; personal fees from Zentiva, and Abbvie; outside the submitted work. Dr Schram declares that the data collection of the primary study by Janssen et al. was supported by unrestricted grants from Janssen, Novo Nordisk, and Sanofi. Dr Wagner declares that she receives personal fees from Celgene, outside the submitted work. All authors declare no other relationships or activities that could appear to have influenced the submitted work. No funder had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Figures


References
-
- Mathers CD, Lopez AD, Murray CJL. The burden of disease and mortality by condition: Data, methods, and results for 2001. In: Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL, eds. Global Burden of Disease and Risk Factors. The International Bank for Reconstruction and Development/The World Bank Group, 2006:45-93. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
