Simplifying TREAtment and Monitoring for HIV (STREAM HIV): protocol for a randomised controlled trial of point-of-care urine tenofovir and viral load testing to improve HIV outcomes
- PMID: 34610939
- PMCID: PMC8493905
- DOI: 10.1136/bmjopen-2021-050116
Simplifying TREAtment and Monitoring for HIV (STREAM HIV): protocol for a randomised controlled trial of point-of-care urine tenofovir and viral load testing to improve HIV outcomes
Abstract
Introduction: Substantial improvements in viral suppression among people living with HIV (PLHIV) are needed to end the HIV epidemic, requiring extensive scale-up of low-cost HIV monitoring services. Point-of-care (POC) tests for monitoring antiretroviral therapy (ART) adherence and viral load (VL) may be efficient and effective tools for real-time clinical decision making. We aim to evaluate the effects of a combined intervention of POC ART adherence and VL testing compared with standard-of-care on ART adherence, viral suppression and retention at 6 and 18 months post-ART initiation among PLHIV.
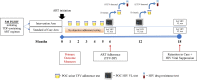
Methods and analysis: Simplifying TREAtment and Monitoring for HIV (STREAM HIV) is a two-arm, open-label, randomised controlled superiority trial of POC urine tenofovir (POC TFV) and VL monitoring in PLHIV. We aim to enrol 540 PLHIV initiating a first-line ART regimen at a public HIV clinic in South Africa. Participants will be randomised 1:1 to the intervention or control arm. Intervention arm participants will receive monthly POC TFV testing for the first 5 months and POC VL testing at months 6 and 12. Intervention arm participants will also receive reflex POC TFV testing if viraemic and reflex HIV drug resistance testing for those with viraemia and detectable TFV. Control arm participants will receive standard-of-care, including laboratory-based VL testing at months 6 and 12. Primary outcomes include ART adherence (TFV-diphosphate concentration) at 6 months and viral suppression and retention at 18 months. Secondary outcomes include viral suppression and retention at 6 months, TFV-diphosphate concentration at 18 months, cost and cost-effectiveness of the intervention and acceptability of the intervention among PLHIV and healthcare workers.
Ethics and dissemination: STREAM HIV has received ethical approval from the University of Washington Institutional Review Board (STUDY00007544), University of KwaZulu-Natal Biomedical Research Ethics Committee (BREC/00000833/2019) and Division of AIDS Regulatory Support Center (38509). Findings will be disseminated at international conferences and in peer-reviewed journals.
Trial registration number: NCT04341779.
Keywords: HIV & AIDS; clinical trials; epidemiology; international health services; public health.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The point-of-care urine tenofovir tests used in this study are provided by Abbott at no cost. The AsantéTM HIV-1 Rapid Recency assays used for exploratory analyses in this study are provided by Sedia Biosciences (Beaverton, Oregon, USA) at no cost.
Figures



References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) . 90–90–90: an ambitious treatment target to help end the AIDS epidemic, 2017. Available: https://www.unaids.org/en/resources/documents/2017/90-90-90 [Accessed 17 Jul 2019].
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) . 2020 Global AIDS Update - Seizing the Moment - Tackling entrenched inequalities to end epidemics, 2020. Available: https://aids2020.unaids.org/report/ [Accessed 13 Nov 2020].
-
- World Health Organization (WHO) . Guidelines on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV, 2015. Available: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ [Accessed 14 Nov 2020]. - PubMed
-
- World Health Organization (WHO) . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, 2016. Available: http://www.who.int/hiv/pub/arv/arv-2016/en/ - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
