Doxorubicin plus lurbinectedin in patients with advanced endometrial cancer: results from an expanded phase I study
- PMID: 34610971
- PMCID: PMC8573419
- DOI: 10.1136/ijgc-2021-002881
Doxorubicin plus lurbinectedin in patients with advanced endometrial cancer: results from an expanded phase I study
Abstract
Objective: Second-line treatment of endometrial cancer is an unmet medical need. We conducted a phase I study evaluating lurbinectedin and doxorubicin intravenously every 3 weeks in patients with solid tumors. The aim of this study was to characterise the efficacy and safety of lurbinectedin and doxorubicin for patients with endometrial cancer.
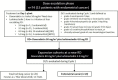
Methods: Thirty-four patients were treated: 15 patients in the escalation phase (doxorubicin 50 mg/m2 and lurbinectedin 3.0-5.0 mg) and 19 patients in the expansion cohort (doxorubicin 40 mg/m2 and lurbinectedin 2.0 mg/m2). All histological subtypes were eligible and patients had received one to two prior lines of chemotherapy for advanced disease. Antitumor activity was evaluated every two cycles according to the Response Evaluation Criteria in Solid Tumors version 1.1. Adverse events were graded according to the National Cancer Institute-Common Terminology Criteria for Adverse Events version 4.
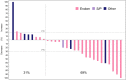
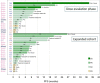
Results: Median age (range) was 65 (51-78) years. Eastern Cooperative Oncology Group performance status was up to 1 in 97% of patients. In the escalation phase, 4 (26.7%) of 15 patients had confirmed response: two complete and two partial responses (95% CI 7.8% to 55.1%). Median duration of response was 19.5 months. Median progression-free survival was 7.3 (2.5 to 10.1) months. In the expansion cohort, confirmed partial response was reported in 8 (42.1%) of 19 patients (95% CI 20.3% to 66.5%). Median duration of response was 7.5 (6.4 to not reached) months, median progression-free survival was 7.7 (2.0 to 16.7) months and median overall survival was 14.2 (4.5 to not reached) months. Fatigue (26.3% of patients), and transient and reversible myelosuppression (neutropenia, 78.9%; febrile neutropenia, 21.1%; thrombocytopenia, 15.8%) were the main grade 3 and higher toxicities in the expanded cohort.
Conclusions: In patients with recurrent advanced endometrial cancer treated with doxorubicin and lurbinectedin, response rates (42%) and duration of response (7.5 months) were favorable. Further evaluation of doxorubicin and lurbinectedin is warranted in this patient population.
Keywords: endometrial neoplasms; endometrium.
© IGCS and ESGO 2021. Re-use permitted under CC BY-NC. No commercial re-use. Published by BMJ.
Conflict of interest statement
Competing interests: RK reports personal fees from Eisai. GSK, Clovis, Basilea, AstraZeneca, Roche, InCyte, and PharmaMar; non-financial support from GSK, Clovis, Basilea, AstraZeneca and Roche; and a grant from Clovis, outside the submitted work. VM reports personal fees and other (travelling support, speaker’s bureau) from Bayer, BMS, Pieris, Roche, Janssen, Regeneron/Sanofi and Nanobiotix, and a grant from Medscape/Bayer, outside the submitted work. VB reports personal fees from Oncoart, Guidepoint, PUMA, Cytomx, Loxo Therapeutics, and institutional financial support for clinical trials from Abbvie, ACEO, Adaptaimmune, Amcure, AMGEN, AstraZeneca, BMS Cytomx, GSK, Genentech/Roche, H3, Incyte, Janssen, Kura, Lilly, Loxo, Nektar, Macrogenics, Menarini, Merck, Merus, Nanobiotix, Novartis, Pfizer, PharmaMar, Principia, PUMA, Sanofi, Taiho, Tesaro, BeiGene, Transgene, Takeda, Incyte, Innovio, MSD, PsiOxus, Seattle Genetics; Mersana; GSK; Daiichi; Nektar; Astellas; ORCA; Boston Therapeutics; Dynavax, DebioPharm, Boehringer Ingelheim, Regeneron, Millennium, Synthon, Spectrum, Rigontec, and Zenith, outside the submitted work. EMG reports advisory/consultancy honorariums from AstraZeneca-MSD, Clovis Oncology, GSK-Tesaro, PharmaMar and Roche; she has received speaker bureau/expert testimony honorariums from AstraZeneca, PharmaMar, Roche and GSK; and has received travel/accommodation/expenses from Roche, Tesaro: A GSK Company and Baxter. IR reports personal fees and non-financial support from Pharmamar; grants, personal fees and non-financial support from Roche; personal fees from Clovis; personal fees from GSK; and grants, personal fees and non-financial support from AstraZeneca, outside the submitted work. EC reports grants and personal fees from Astellas, Novartis, Nanobiotix, Pfizer, Janssen-Cilag, PsiOxus Therapeutics, Merck, BMS, Seattle Genetics, Boehringer Ingelheim, AstraZeneca, Roche/Genentech, Servier, Celgene, Abbvie, Amcure, Alkermes, PharmaMar, BeiGene and GLG; personal fees from Medscape, Gilead, Pierre Fabre, Cerulean Pharma, EUSA, Gerhmann Consulting, Guidepoint, OncoDNA; and grants from ACEO, Adaptaimmune, AMGEN, Cytomx, GSK, H3, Incyte, Kura, Lilly, Nektar, Loxo, Macrogenics, Menarini, Merus, Principia, PUMA, Sanofi, Taiho, Tesaro, Transgene, Takeda, Innovio, MSD, Mersana, Daiichi, ORCA, Boston Therapeutics, Dynavax, DebioPharm, Regeneron, Millenium, Synthon, Spectrum and Rigontec, outside the submitted work. NB reports personal fees and non-financial support from BMS and Merck Serono, and personal fees from AstraZeneca, BioNTech, Eisai and MSD, outside the submitted work. CK, JAL-V, MS, VA and AZ report grants from the Centro para el Desarrollo Tecnológico Industrial (CDTI) during the conduct of the study; and personal fees for salary as full time employee and stock ownership from Pharma Mar, outside the submitted work. MF reports grants from Merck Educational Grant for EACH study, grants from MSD Educational Grant for POPPY study, grants from Boehringer Ingelheim Educational Grant for DARWIN1 study, grants from AstraZeneca Educational Grant for ORCA2 study, personal fees from Achilles Advisory and Consultancy Work, personal fees from AstraZeneca Advisory and Consultancy Work, personal fees from BMS Advisory and Consultancy Work, personal fees from Merck Advisory and Consultancy Work, personal fees from MSD Advisory and Consultancy Work, personal fees from Nanobiotix Advisory and Consultancy Work, personal fees from Novartis Advisory and Consultancy Work, personal fees from Pfizer Advisory and Consultancy Work, personal fees from Pharmamar Advisory and Consultancy Work, personal fees from Roche Advisory and Consultancy Work, personal fees from Takeda Advisory and Consultancy Work, personal fees from Bayer Advisory and Consultancy Work, outside the submitted work.
Figures
References
-
- Makker V, Hensley ML, Zhou Q, et al. . Treatment of advanced or recurrent endometrial carcinoma with doxorubicin in patients progressing after paclitaxel/carboplatin: Memorial Sloan-Kettering cancer center experience from 1995 to 2009. Int J Gynecol Cancer 2013;23:929–34. 10.1097/IGC.0b013e3182915c20 - DOI - PMC - PubMed
-
- NCCN clinical practice guidelines in oncology (NCCN guidelines): uterine neoplasms. version 03, 2021. Available: http://wwwnccnorg/professionals/defaultaspx
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

