Associations of Disease-Modifying Therapies With COVID-19 Severity in Multiple Sclerosis
- PMID: 34610987
- PMCID: PMC8601210
- DOI: 10.1212/WNL.0000000000012753
Associations of Disease-Modifying Therapies With COVID-19 Severity in Multiple Sclerosis
Abstract
Background and objectives: People with multiple sclerosis (MS) are a vulnerable group for severe coronavirus disease 2019 (COVID-19), particularly those taking immunosuppressive disease-modifying therapies (DMTs). We examined the characteristics of COVID-19 severity in an international sample of people with MS.
Methods: Data from 12 data sources in 28 countries were aggregated (sources could include patients from 1-12 countries). Demographic (age, sex), clinical (MS phenotype, disability), and DMT (untreated, alemtuzumab, cladribine, dimethyl fumarate, glatiramer acetate, interferon, natalizumab, ocrelizumab, rituximab, siponimod, other DMTs) covariates were queried, along with COVID-19 severity outcomes, hospitalization, intensive care unit (ICU) admission, need for artificial ventilation, and death. Characteristics of outcomes were assessed in patients with suspected/confirmed COVID-19 using multilevel mixed-effects logistic regression adjusted for age, sex, MS phenotype, and Expanded Disability Status Scale (EDSS) score.
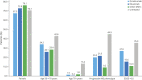
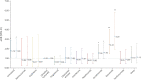
Results: Six hundred fifty-seven (28.1%) with suspected and 1,683 (61.9%) with confirmed COVID-19 were analyzed. Among suspected plus confirmed and confirmed-only COVID-19, 20.9% and 26.9% were hospitalized, 5.4% and 7.2% were admitted to ICU, 4.1% and 5.4% required artificial ventilation, and 3.2% and 3.9% died. Older age, progressive MS phenotype, and higher disability were associated with worse COVID-19 outcomes. Compared to dimethyl fumarate, ocrelizumab and rituximab were associated with hospitalization (adjusted odds ratio [aOR] 1.56, 95% confidence interval [CI] 1.01-2.41; aOR 2.43, 95% CI 1.48-4.02) and ICU admission (aOR 2.30, 95% CI 0.98-5.39; aOR 3.93, 95% CI 1.56-9.89), although only rituximab was associated with higher risk of artificial ventilation (aOR 4.00, 95% CI 1.54-10.39). Compared to pooled other DMTs, ocrelizumab and rituximab were associated with hospitalization (aOR 1.75, 95% CI 1.29-2.38; aOR 2.76, 95% CI 1.87-4.07) and ICU admission (aOR 2.55, 95% CI 1.49-4.36; aOR 4.32, 95% CI 2.27-8.23), but only rituximab was associated with artificial ventilation (aOR 6.15, 95% CI 3.09-12.27). Compared to natalizumab, ocrelizumab and rituximab were associated with hospitalization (aOR 1.86, 95% CI 1.13-3.07; aOR 2.88, 95% CI 1.68-4.92) and ICU admission (aOR 2.13, 95% CI 0.85-5.35; aOR 3.23, 95% CI 1.17-8.91), but only rituximab was associated with ventilation (aOR 5.52, 95% CI 1.71-17.84). Associations persisted on restriction to confirmed COVID-19 cases. No associations were observed between DMTs and death. Stratification by age, MS phenotype, and EDSS score found no indications that DMT associations with COVID-19 severity reflected differential DMT allocation by underlying COVID-19 severity.
Discussion: Using the largest cohort of people with MS and COVID-19 available, we demonstrated consistent associations of rituximab with increased risk of hospitalization, ICU admission, and need for artificial ventilation and of ocrelizumab with hospitalization and ICU admission. Despite the cross-sectional design of the study, the internal and external consistency of these results with prior studies suggests that rituximab/ocrelizumab use may be a risk factor for more severe COVID-19.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures


Comment in
-
B-Cell Depletion and COVID-19 Severity in Multiple Sclerosis: Remaining Challenges.Neurology. 2021 Nov 9;97(19):885-886. doi: 10.1212/WNL.0000000000012754. Epub 2021 Oct 5. Neurology. 2021. PMID: 34610986 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
