Targeting the miRNA-155/TNFSF10 network restrains inflammatory response in the retina in a mouse model of Alzheimer's disease
- PMID: 34611142
- PMCID: PMC8492692
- DOI: 10.1038/s41419-021-04165-x
Targeting the miRNA-155/TNFSF10 network restrains inflammatory response in the retina in a mouse model of Alzheimer's disease
Abstract
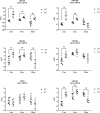
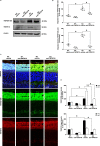
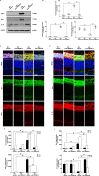
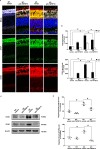
Age-related disorders, such as Alzheimer's disease (AD) and age-related macular degeneration (AMD) share common features such as amyloid-β (Aβ) protein accumulation. Retinal deposition of Aβ aggregates in AMD patients has suggested a potential link between AMD and AD. In the present study, we analyzed the expression pattern of a focused set of miRNAs, previously found to be involved in both AD and AMD, in the retina of a triple transgenic mouse model of AD (3xTg-AD) at different time-points. Several miRNAs were differentially expressed in the retina of 3xTg-AD mice, compared to the retina of age-matched wild-type (WT) mice. In particular, bioinformatic analysis revealed that miR-155 had a central role in miRNA-gene network stability, regulating several pathways, including apoptotic and inflammatory signaling pathways modulated by TNF-related apoptosis-inducing ligand (TNFSF10). We showed that chronic treatment of 3xTg-AD mice with an anti-TNFSF10 monoclonal antibody was able to inhibit the retinal expression of miR-155, which inversely correlated with the expression of its molecular target SOCS-1. Moreover, the fine-tuned mechanism related to TNFSF10 immunoneutralization was tightly linked to modulation of TNFSF10 itself and its death receptor TNFRSF10B, along with cytokine production by microglia, reactive gliosis, and specific AD-related neuropathological hallmarks (i.e., Aβ deposition and Tau phosphorylation) in the retina of 3xTg-AD mice. In conclusion, immunoneutralization of TNFSF10 significantly preserved the retinal tissue in 3xTg-AD mice, suggesting its potential therapeutic application in retinal degenerative disorders.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Czakó C, Kovács T, Ungvari Z, Csiszar A, Yabluchanskiy A, Conley S, et al. Retinal biomarkers for Alzheimer’s disease and vascular cognitive impairment and dementia (VCID): implication for early diagnosis and prognosis. GeroScience. 2020;42:1499–525. doi: 10.1007/s11357-020-00252-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

