Ganitumab and metformin plus standard neoadjuvant therapy in stage 2/3 breast cancer
- PMID: 34611148
- PMCID: PMC8492731
- DOI: 10.1038/s41523-021-00337-2
Ganitumab and metformin plus standard neoadjuvant therapy in stage 2/3 breast cancer
Abstract
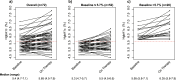
I-SPY2 is an adaptively randomized phase 2 clinical trial evaluating novel agents in combination with standard-of-care paclitaxel followed by doxorubicin and cyclophosphamide in the neoadjuvant treatment of breast cancer. Ganitumab is a monoclonal antibody designed to bind and inhibit function of the type I insulin-like growth factor receptor (IGF-1R). Ganitumab was tested in combination with metformin and paclitaxel (PGM) followed by AC compared to standard-of-care alone. While pathologic complete response (pCR) rates were numerically higher in the PGM treatment arm for hormone receptor-negative, HER2-negative breast cancer (32% versus 21%), this small increase did not meet I-SPY's prespecified threshold for graduation. PGM was associated with increased hyperglycemia and elevated hemoglobin A1c (HbA1c), despite the use of metformin in combination with ganitumab. We evaluated several putative predictive biomarkers of ganitumab response (e.g., IGF-1 ligand score, IGF-1R signature, IGFBP5 expression, baseline HbA1c). None were specific predictors of response to PGM, although several signatures were associated with pCR in both arms. Any further development of anti-IGF-1R therapy will require better control of anti-IGF-1R drug-induced hyperglycemia and the development of more predictive biomarkers.
© 2021. The Author(s).
Conflict of interest statement
Doug Yee has received unrelated research support from Boehringer Ingleheim. Claudine Isaacs has received consulting fees from Seattle Genetics, Genentech, AstraZeneca, Novartis, PUMA, Pfizer, and Esai. Christina Yau has consulted for NantOmics, LLC, part of the NantWorks network. Paul Haluska was employed at BMS after the work was conducted on this paper and currently holds stock options. A. Jo Chein reports institutional research support from Seagen, Merck, Amgen and Puma Biotechnology. Lajos Pusztai has received consulting fees and honoraria from Seattle Genetics, Pfizer, Astra Zeneca, Merck, Novartis, Bristol-Myers Squibb, Pfizer, Genentech, Eisai, Pieris, Immunomedics, Clovis, Syndax, H3Bio, Radius Health, and Daiichi, institutional research support from Seattle Genetics, AstraZeneca, Merck, Pfizer and Bristol Myers Squibb. Barbara Haley reports institutional support from Pfizer, Lilly, Daiichi Sankyo, Roche, Puma, Astra Zeneca and Sanofi. Judy C. Boughey has received support from Eli Lilly. Debu Tripathy has received support from Novartis, Pfizer, AstraZeneca, GSK and Immunomedics. Amy S. Clark has received unrelated research support from Novartis. Hyo S. Han reports institutional support from GlaxoSmithKline, Abbvie, Prescient, G1 therapeutics, Marker Therapeutics, Novartis, Horizon Pharma, Pfizer, Seattle Genetics, Arvinas and Zymeworks, and is part of Lilly’s speaker’s bureau. E.F. Petricoin reports: leadership roles with: Perthera and Ceres Nanosciences; stock or other ownership interests in Perthera, Ceres Nanosciences and Avant Diagnostics; consulting or advisory roles with Perthera, Ceres Nanosciences, AZGen, Avant Diagnostics; institutional research support from Ceres Nanosciences, GlaxoSmithKline), Abbvie, Symphogen, and Genentech; patents, royalties, other intellectual property from National Institutes of Health patents licensing fee distribution/royalty, co-inventor on filed George Mason University–assigned patents related to phosphorylated HER2 and EGFR response predictors for HER family-directed therapeutics, as such can receive royalties and licensing distribution on any licensed IP; travel, accommodations, expenses received from Perthera and Ceres Nanosciences. Erica Stringer-Reasor reports receiving research funds or consulting fees from Lilly, Immunomedics and Mylan. J.D. Wulfkuhle received honoraria from DAVA Oncology and consults for Baylor College of Medicine. Angela DeMichele has received honoraria or consulting fees from Pfizer and Context Therapeutics and reports institutional research support from Novartis, Pfizer, Genentech, Calithera and Menarini. Michelle Melisko has consulted for Biotheranostics and reports institutional research support from AstraZeneca, Novartis, KCRN Research and Puma Biotechnology. Hope Rugo reports institutional research support from Pfizer, Merck, Novartis, Lilly, Genentech, Odonate, Daiichi, Seattle Genetics, Eisai, Macrogenics, Sermonix, Boehringer Ingelheim, Polyphor, Astra Zeneca and Immunomedics, and has received honoraria from Puma Biotechnology, Mylan and Samsung. Laura J. van ‘t Veer is a part-time employee and stockholder in Agendia N.V. Laura Esserman is an unpaid member of the board of directors of Quantum Leap Healthcare Collaborative (QLHC) and received grant support from QLHC for the I-SPY2 Trial; she is a member of the Blue Cross/Blue Shield Medical Advisory Panel and receives reimbursement for her time and travel; Dr. Esserman has received unrelated research support from Merck. The remaining authors declare no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

