Afucosylated Plasmodium falciparum-specific IgG is induced by infection but not by subunit vaccination
- PMID: 34611164
- PMCID: PMC8492741
- DOI: 10.1038/s41467-021-26118-w
Afucosylated Plasmodium falciparum-specific IgG is induced by infection but not by subunit vaccination
Abstract
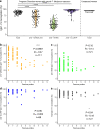
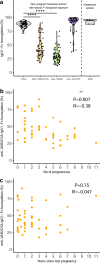
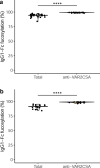
Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) family members mediate receptor- and tissue-specific sequestration of infected erythrocytes (IEs) in malaria. Antibody responses are a central component of naturally acquired malaria immunity. PfEMP1-specific IgG likely protects by inhibiting IE sequestration and through IgG-Fc Receptor (FcγR) mediated phagocytosis and killing of antibody-opsonized IEs. The affinity of afucosylated IgG to FcγRIIIa is up to 40-fold higher than fucosylated IgG, resulting in enhanced antibody-dependent cellular cytotoxicity. Most IgG in plasma is fully fucosylated, but afucosylated IgG is elicited in response to enveloped viruses and to paternal alloantigens during pregnancy. Here we show that naturally acquired PfEMP1-specific IgG is strongly afucosylated in a stable and exposure-dependent manner, and efficiently induces FcγRIIIa-dependent natural killer (NK) cell degranulation. In contrast, immunization with a subunit PfEMP1 (VAR2CSA) vaccine results in fully fucosylated specific IgG. These results have implications for understanding protective natural- and vaccine-induced immunity to malaria.
Trial registration: ClinicalTrials.gov NCT02647489.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World Health Organization. World Malaria Report 2020 (2020).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

