Low-pressure chromatographic separation and UV/Vis spectrophotometric characterization of the native and desialylated human apo-transferrin
- PMID: 34611562
- PMCID: PMC8477197
- DOI: 10.1016/j.heliyon.2021.e08030
Low-pressure chromatographic separation and UV/Vis spectrophotometric characterization of the native and desialylated human apo-transferrin
Abstract
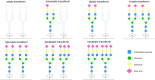
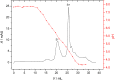
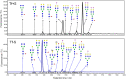
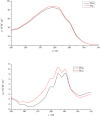
Low-pressure pH gradient ion exchange separation provides a fast, simple and cost-effective method for preparative purification of native and desialylated apo-transferrin. The method enables easy monitoring of the extent of the desialylation reaction and also the efficient separation and purification of protein fractions after desialylation. The N-glycan analysis shows that the modified desialylation protocol successfully reduces the content of the sialylated fractions relative to the native apo-transferrin. In the optimized protocol, the desialylation capacity is increased by 150 %, compared to the original protocol provided by the manufacturer. The molar absorption coefficients in the near-UV region for the native and desialylated apo-transferrin differ by several percent, suggesting a subtle dependence of the glycoprotein absorbance on the variable sialic acid content. The method can easily be modified for other glycoproteins and is particularly appropriate for quick testing of sialic acid content in the protein glycosylation patterns prior to further verification by mass spectrometry.
Keywords: Chromatography; Glycoforms; Molar absorbance; Sialic acid; Transferrin; UV-Vis spectroscopy.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Bertozzi C.R., Hart G.W., Etzler M.E., editors. Essentials of Glycobiology. second ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2009. http://www.ncbi.nlm.nih.gov/books/NBK1908/ - PubMed
-
- Alper J. Searching for medicine’s sweet spot. Science. 2001;291:2338–2343. - PubMed
-
- Kobata A. A retrospective and prospective view of glycopathology. Glycoconj. J. 1998;15:323–331. - PubMed
-
- Stibler H. Carbohydrate-deficient transferrin in serum: a new marker of potentially harmful alcohol consumption reviewed. Clin. Chem. 1991;37:2029–2037. - PubMed
LinkOut - more resources
Full Text Sources

