Sleeping ribosomes: Bacterial signaling triggers RaiA mediated persistence to aminoglycosides
- PMID: 34611612
- PMCID: PMC8476650
- DOI: 10.1016/j.isci.2021.103128
Sleeping ribosomes: Bacterial signaling triggers RaiA mediated persistence to aminoglycosides
Abstract
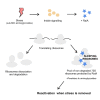
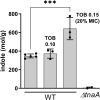
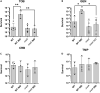
Indole is a molecule proposed to be involved in bacterial signaling. We find that indole secretion is induced by sublethal tobramycin concentrations and increases persistence to aminoglycosides in V. cholerae. Indole transcriptomics showed increased expression of raiA, a ribosome associated factor. Deletion of raiA abolishes the appearance of indole dependent persisters to aminoglycosides, although its overexpression leads to 100-fold increase of persisters, and a reduction in lag phase, evocative of increased active 70S ribosome content, confirmed by sucrose gradient analysis. We propose that, under stress conditions, RaiA-bound inactive 70S ribosomes are stored as "sleeping ribosomes", and are rapidly reactivated upon stress relief. Our results point to an active process of persister formation through ribosome protection during translational stress (e.g., aminoglycoside treatment) and reactivation upon antibiotic removal. Translation is a universal process, and these results could help elucidate a mechanism of persistence formation in a controlled, thus inducible way.
Keywords: Bacteriology; Microbial genomics; Viral microbiology.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Andersson D.I., Hughes D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014;12:465–478. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

