This is a preprint.
Anti-PF4 VITT antibodies are oligoclonal and variably inhibited by heparin
- PMID: 34611669
- PMCID: PMC8491860
- DOI: 10.1101/2021.09.23.21263047
Anti-PF4 VITT antibodies are oligoclonal and variably inhibited by heparin
Update in
-
Monoclonal and oligoclonal anti-platelet factor 4 antibodies mediate VITT.Blood. 2022 Jul 7;140(1):73-77. doi: 10.1182/blood.2021014588. Blood. 2022. PMID: 35560046 Free PMC article.
Abstract
Background: COVID-19 vaccines have been associated with a rare thrombotic and thrombocytopenic reaction, Vaccine-induced immune thrombotic thrombocytopenia (VITT) characterized by platelet-activating anti-PF4 antibodies. This study sought to assess clonality of VITT antibodies and evaluate their characteristics in antigen-based and functional platelet studies.
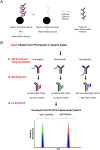
Methods: Anti-PF4 antibodies were isolated from five patients with VITT secondary to ChAdOx1 nCoV-19 (n=1) or Ad26.COV2.S (n=4) vaccination. For comparative studies with heparin-induced thrombocytopenia (HIT), anti-PF4 antibodies were isolated from one patient with spontaneous HIT, another with "classical" HIT, and two patients with non-pathogenic (non-platelet activating) anti-PF4 antibodies. Isolated antibodies were subject to ELISA and functional testing, and mass spectrometric evaluation for clonality determination.
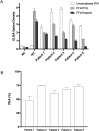
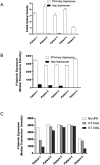
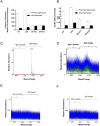
Results: All five VITT patients had oligoclonal anti-PF4 antibodies (3 monoclonal, one bi- and one tri-clonal antibodies), while HIT anti-PF4 antibodies were polyclonal. Notably, like VITT antibodies, anti-PF4 antibodies from a spontaneous HIT patient were monoclonal. The techniques employed did not detect non-pathogenic anti-PF4 antibodies. The ChAdOx1 nCoV-19-associated VITT patient made an excellent recovery with heparin treatment. In vitro studies demonstrated strong inhibition of VITT antibody-induced platelet activation with therapeutic concentrations of heparin in this and one Ad26.COV2.S-associated VITT patient. Oligoclonal VITT antibodies with persistent platelet-activating potential were detected at 6 and 10 weeks after acute presentation in two patients tested. Two of the 5 VITT patients had recurrence of thrombocytopenia and one patient had focal seizures several weeks after acute presentation.
Conclusion: Oligoclonal anti-PF4 antibodies mediate VITT. Heparin use in VITT needs to be further studied.
Conflict of interest statement
Conflict-of-interest disclosure:
AP reports pending/issued patents (Mayo Clinic, Retham Technologies and Versiti BloodCenter of Wisconsin), equity ownership in Retham Technologies, and serving on the advisory board of Veralox Therapeutics. DM reports pending/issued patents (Mayo Clinic). The remaining authors declare no competing financial interests.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
