Idiopathic pulmonary fibrosis: Physician and patient perspectives on the pathway to care from symptom recognition to diagnosis and disease burden
- PMID: 34611971
- PMCID: PMC9135122
- DOI: 10.1111/resp.14154
Idiopathic pulmonary fibrosis: Physician and patient perspectives on the pathway to care from symptom recognition to diagnosis and disease burden
Abstract
Background and objective: Idiopathic pulmonary fibrosis (IPF) is a chronic progressive disease that requires ongoing care and is associated with considerable socioeconomic burden. We evaluated the IPF care pathway from symptom recognition to treatment. We describe the impact of IPF on healthcare resource use (HCRU), quality of life (QoL) and work impairment, and report differences in patient and physician perspectives using real-world data from France, Germany, Japan and the United States.
Methods: Quantitative, point-in-time data were collected as part of the Adelphi IPF II Disease Specific Programme™. Physician-reported data (patient demographics, medical history, diagnoses, treatment) were matched to patient-reported data (HCRU, QoL, work impairment). HCRU was measured as physician visits and hospitalizations. QoL and work impairment were measured using the EuroQol-5 Dimensions (EQ-5D) and Work Productivity and Activity Impairment questionnaires.
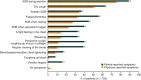
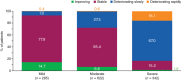
Results: Overall, 244 physicians reported data on 1249 patients, 739 of whom self-reported data. Diagnostic delays of 0.8 (Germany) to 2.0 (Japan) years after symptom onset were reported; treatment initiation was further delayed. In all countries, patients more often reported symptoms in the survey than did their physicians. On average, patients underwent 7-10 clinical tests before diagnosis. Antifibrotic use increased from 57% (2016) to 69% (2019); only 50% of patients with moderate/severe IPF were satisfied with their treatment. The 12-month hospitalization rates were 24% (Japan) to 64% (United States). Patients reported low QoL (mean EQ-5D visual analogue scale: 61.7/100).
Conclusion: Patients with IPF experience considerable diagnostic and treatment delays. More effective therapies and management are needed to reduce the disease burden.
Keywords: interstitial lung disease; pulmonary fibrosis; quality of life; rare lung diseases; respiratory lung function tests.
© 2021 Galapagos NV. Respirology published by John Wiley & Sons Australia, Ltd on behalf of Asian Pacific Society of Respirology.
Conflict of interest statement
This study was also previously presented at the 2020 Annual Congress of the European Respiratory Society. The research was funded by Galapagos NV (Mechelen, Belgium). The study sponsor, Galapagos NV (Mechelen, Belgium), played a role in the study design, data collection and analysis; decision to publish; and preparation of the manuscript. Medical writing support, including development of a draft outline and subsequent drafts in consultation with the authors, collating author comments, copyediting, fact checking and referencing, was provided by Kristian Clausen, MPH at Aspire Scientific Limited (Bollington, UK). Funding for medical writing support for this article was provided by Galapagos NV (Mechelen, Belgium).
Lisa Lancaster has received grant/research support from Bellerophon, Biogen, Boehringer Ingelheim, Celgene, Galactic, Galapagos NV, Genentech, Novartis, BMS, Fibrogen and Pliant; has worked as a paid consultant for AstraZeneca, DevProBiopharma, Galapagos NV, Genentech, United Therapeutics and Veracyte; and has been paid as a speaker (disease state education) by Boehringer Ingelheim, Genentech, United Therapeutics and Veracyte. Francesco Bonella has received grant/research support from Boehringer Ingelheim and Savara; has worked as a paid consultant for Boehringer Ingelheim, Bristol Myers Squibb, Galapagos, Roche and Savara; and has been paid as a speaker by Boehringer Ingelheim and Roche. Yoshikazu Inoue has worked as a paid consultant for Asahi Kasei, Boehringer Ingelheim, Galapagos, NITTO, Promedior, Roche, Shionogi and Taiho, and unpaid advisor for Savara; and has been paid as a speaker by Boehringer Ingelheim and Shionogi. Vincent Cottin has received grant/research support from Boehringer Ingelheim; has worked as a paid consultant for Roche/Promedior; has participated in advisory boards for Actelion, Bayer/MSD, Galapagos and Novartis; has received payment for travel to medical meetings from Actelion, Boehringer Ingelheim and Roche; has been paid as a speaker by Actelion, Boehringer Ingelheim, Novartis, Roche and Sanofi; and has served as a member/chair of data safety monitoring boards for Bayer/MSD, Celgene, Galapagos, Galecto and Promedior. Jonathan Langley is an employee and warrant holder at Galapagos NV. Mark Small and James Siddall are employees of Adelphi Real World, a company that received research funding from Galapagos NV for the current study.
Figures


Comment in
-
Delays in idiopathic pulmonary fibrosis diagnosis and treatment: Time for change.Respirology. 2022 Jan;27(1):10-11. doi: 10.1111/resp.14189. Epub 2021 Nov 30. Respirology. 2022. PMID: 34846086 No abstract available.
References
-
- Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

