Effect of Long-Term Marine ɷ-3 Fatty Acids Supplementation on the Risk of Atrial Fibrillation in Randomized Controlled Trials of Cardiovascular Outcomes: A Systematic Review and Meta-Analysis
- PMID: 34612056
- PMCID: PMC9109217
- DOI: 10.1161/CIRCULATIONAHA.121.055654
Effect of Long-Term Marine ɷ-3 Fatty Acids Supplementation on the Risk of Atrial Fibrillation in Randomized Controlled Trials of Cardiovascular Outcomes: A Systematic Review and Meta-Analysis
Abstract
Background: Some, but not all, large-scale randomized controlled trials (RCTs) investigating the effects of marine ɷ-3 fatty acids supplementation on cardiovascular outcomes have reported increased risks of atrial fibrillation (AF). The potential reasons for disparate findings may be dose-related.
Methods: The MEDLINE and Embase databases were searched for articles and abstracts published between January 1, 2012, and December 31, 2020, in addition to a meta-analysis of large cardiovascular RCTs published in 2019. RCTs of cardiovascular outcomes of marine ɷ-3 fatty acids that reported results for AF, either as a prespecified outcome, an adverse event, or a cause for hospitalization, with a minimum sample size of 500 patients and a median follow-up of at least 1 year were included. RCTs specifically examining shorter-term effects of ɷ-3 fatty acids on recurrent AF in patients with established AF or postoperative AF were not included. The hazard ratio (HR) for the reported AF outcomes within each trial was meta-analyzed using random effects model with Knapp-Hartung adjustment and evaluated a dose-response relationship with a meta-regression model.
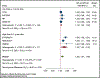
Results: Of 4049 screened records, 7 studies were included in the meta-analysis. Of those, 5 were already detected in a previous meta-analysis of cardiovascular RCTs. Among the 81 210 patients from 7 trials, 58 939 (72.6%) were enrolled in trials testing ≤1 g/d and 22 271 (27.4%) in trials testing >1 g/d of ɷ-3 fatty acids. The mean age was 65 years, and 31 842 (39%) were female. The weighted average follow-up was 4.9 years. In meta-analysis, the use of marine ɷ-3 fatty acid supplements was associated with an increased risk of AF (n=2905; HR, 1.25 [95% CI, 1.07-1.46]; P=0.013). In analyses stratified by dose, the HR was greater in the trials testing >1 g/d (HR, 1.49 [95% CI, 1.04-2.15]; P=0.042) compared with those testing ≤1 g/d (HR, 1.12 [95% CI, 1.03-1.22]; P=0.024; P for interaction <0.001). In meta-regression, the HR for AF increased per 1 g higher dosage of ɷ-3 fatty acids dosage (HR, 1.11 [95% CI, 1.06-1.15]; P=0.001).
Conclusions: In RCTs examining cardiovascular outcomes, marine ɷ-3 supplementation was associated with an increased risk of AF. The risk appeared to be greater in trials testing >1 g/d.
Keywords: atrial fibrillation; clinical trials; dietary supplements; fatty acids, omega-3; meta-analysis.
Figures


Comment in
-
Fish Oil Supplements May Increase the Risk for Atrial Fibrillation: What Does This Mean?Circulation. 2021 Dec 21;144(25):1991-1994. doi: 10.1161/CIRCULATIONAHA.121.057464. Epub 2021 Dec 20. Circulation. 2021. PMID: 34928703 No abstract available.
-
Do Fish Oil Supplements Reduce A-Fib?Mo Med. 2022 Nov-Dec;119(6):543-544. Mo Med. 2022. PMID: 36588640 Free PMC article. No abstract available.
References
-
- Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CMand Investigators R-I. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019;380:11–22. - PubMed
-
- Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–55. - PubMed
-
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K and Japan EPAlisI. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8. - PubMed
-
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O and Group ESCSD. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. - PubMed

