Pathogenicity and virulence of Listeria monocytogenes: A trip from environmental to medical microbiology
- PMID: 34612177
- PMCID: PMC8496543
- DOI: 10.1080/21505594.2021.1975526
Pathogenicity and virulence of Listeria monocytogenes: A trip from environmental to medical microbiology
Abstract
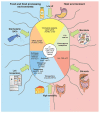
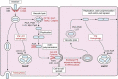
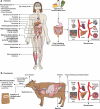
Listeria monocytogenes is a saprophytic gram-positive bacterium, and an opportunistic foodborne pathogen that can produce listeriosis in humans and animals. It has evolved an exceptional ability to adapt to stress conditions encountered in different environments, resulting in a ubiquitous distribution. Because some food preservation methods and disinfection protocols in food-processing environments cannot efficiently prevent contaminations, L. monocytogenes constitutes a threat to human health and a challenge to food safety. In the host, Listeria colonizes the gastrointestinal tract, crosses the intestinal barrier, and disseminates through the blood to target organs. In immunocompromised individuals, the elderly, and pregnant women, the pathogen can cross the blood-brain and placental barriers, leading to neurolisteriosis and materno-fetal listeriosis. Molecular and cell biology studies of infection have proven L. monocytogenes to be a versatile pathogen that deploys unique strategies to invade different cell types, survive and move inside the eukaryotic host cell, and spread from cell to cell. Here, we present the multifaceted Listeria life cycle from a comprehensive perspective. We discuss genetic features of pathogenic Listeria species, analyze factors involved in food contamination, and review bacterial strategies to tolerate stresses encountered both during food processing and along the host's gastrointestinal tract. Then we dissect host-pathogen interactions underlying listerial pathogenesis in mammals from a cell biology and systemic point of view. Finally, we summarize the epidemiology, pathophysiology, and clinical features of listeriosis in humans and animals. This work aims to gather information from different fields crucial for a comprehensive understanding of the pathogenesis of L. monocytogenes.
Keywords: Listeriosis; food contamination; intracellular pathogen; pathogenesis; stress response.
Figures




References
-
- World Organisation for Animal Health . Access online: OIE - World Organisation for Animal Health [Internet]. 2018. cited 2020 Jul 31. Available from: https://www.oie.int/en/standard-setting/terrestrial-manual/access-online/.
-
- Quereda JJ, Leclercq A, Moura A, et al. Listeria valentina sp. nov., isolated from a water trough and the faeces of healthy sheep. Int J Syst Evol Microbiol. 2020;70:5868–5879. - PubMed
-
- Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16:32–46. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
