Mesenchymal Stromal Cells: an Antimicrobial and Host-Directed Therapy for Complex Infectious Diseases
- PMID: 34612662
- PMCID: PMC8510528
- DOI: 10.1128/CMR.00064-21
Mesenchymal Stromal Cells: an Antimicrobial and Host-Directed Therapy for Complex Infectious Diseases
Abstract
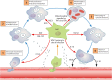
There is an urgent need for new antimicrobial strategies for treating complex infections and emerging pathogens. Human mesenchymal stromal cells (MSCs) are adult multipotent cells with antimicrobial properties, mediated through direct bactericidal activity and modulation of host innate and adaptive immune cells. More than 30 in vivo studies have reported on the use of human MSCs for the treatment of infectious diseases, with many more studies of animal MSCs in same-species models of infection. MSCs demonstrate potent antimicrobial effects against the major classes of human pathogens (bacteria, viruses, fungi, and parasites) across a wide range of infection models. Mechanistic studies have yielded important insight into their immunomodulatory and bactericidal activity, which can be enhanced through various forms of preconditioning. MSCs are being investigated in over 80 clinical trials for difficult-to-treat infectious diseases, including sepsis and pulmonary, intra-abdominal, cutaneous, and viral infections. Completed trials consistently report MSCs to be safe and well tolerated, with signals of efficacy against some infectious diseases. Although significant obstacles must be overcome to produce a standardized, affordable, clinical-grade cell therapy, these studies suggest that MSCs may have particular potential as an adjunct therapy in complex or resistant infections.
Keywords: antimicrobial; cell therapy; clinical trials; host-directed therapy; immunomodulation; immunotherapy; infectious diseases; mesenchymal stromal cells; pathogens.
Figures


References
-
- Chambers HF, Bartlett JG, Bonomo RA, Chiou C, Cosgrove SE, Cross HR, Daum RS, Downing M, Evans SR, Knisely J, Kreiswirth BN, Lautenbach E, Mickley BS, Patel R, Pettigrew MM, Rodvold KA, Spellberg B, Fowler VG. 2014. Antibacterial Resistance Leadership Group: open for business. Clin Infect Dis 58:1571–1576. 10.1093/cid/ciu132. - DOI - PMC - PubMed
-
- World Health Organization. 2016. Global action plan on antimicrobial resistance. World Health Organization, Geneva, Switzerland. https://www.who.int/publications/i/item/9789241509763.
-
- Zumla A, Rao M, Wallis RS, Kaufmann SHE, Rustomjee R, Mwaba P, Vilaplana C, Yeboah-Manu D, Chakaya J, Ippolito G, Azhar E, Hoelscher M, Maeurer M, Host-Directed Therapies Network Consortium. 2016. Host-directed therapies for infectious diseases: current status, recent progress, and future prospects. Lancet Infect Dis 16:e47–e63. 10.1016/S1473-3099(16)00078-5. - DOI - PMC - PubMed
-
- Zumla A, Memish ZA, Maeurer M, Bates M, Mwaba P, Al-Tawfiq JA, Denning DW, Hayden FG, Hui DS. 2014. Emerging novel and antimicrobial-resistant respiratory tract infections: new drug development and therapeutic options. Lancet Infect Dis 14:1136–1149. 10.1016/S1473-3099(14)70828-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Department of Health, Northern Ireland
- MR/R025096/1/MRC_/Medical Research Council/United Kingdom
- National Institute for Health Research (NIHR)
- Insmed
- Fundação Champalimaud (CF)
- WT_/Wellcome Trust/United Kingdom
- The Wellcome Trust
- European and Developing Countries Clinical Trials Partnership (EDCTP)
- MR/S009426/1/MRC_/Medical Research Council/United Kingdom
- MR/R017867/1/MRC_/Medical Research Council/United Kingdom
- Bill and Melinda Gates Foundation (BMGF)
- UKRI | Medical Research Council (MRC)
LinkOut - more resources
Full Text Sources
Medical

