A Serological Snapshot of COVID-19 Initial Stages in Israel by a 6-Plex Antigen Array
- PMID: 34612689
- PMCID: PMC8510178
- DOI: 10.1128/Spectrum.00870-21
A Serological Snapshot of COVID-19 Initial Stages in Israel by a 6-Plex Antigen Array
Abstract
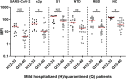
The first case of SARS-CoV-2 was discovered in Israel in late February 2020. Three major outbreaks followed, resulting in over 800,000 cases and over 6,000 deaths by April 2021. Our aim was characterization of a serological snapshot of Israeli patients and healthy adults in the early months of the COVID-19 pandemic. Sera from 55 symptomatic COVID-19 patients and 146 healthy subjects (early-pandemic, reverse transcription-quantitative PCR [qRT-PCR]-negative), collected in Israel between March and April 2020, were screened for SARS-CoV-2-specific IgG, IgM, and IgA antibodies, using a 6-plex antigen microarray presenting the whole inactivated virus and five viral antigens: a stabilized version of the spike ectodomain (S2P), spike subunit 1 (S1), receptor-binding-domain (RBD), N-terminal-domain (NTD), and nucleocapsid (NC). COVID-19 patients, 4 to 40 days post symptom onset, presented specific IgG to all of the viral antigens (6/6) in 54 of the 55 samples (98% sensitivity). Specific IgM and IgA antibodies for all six antigens were detected in only 10% (5/55) and 4% (2/55) of the patients, respectively, suggesting that specific IgG is a superior serological marker for COVID-19. None of the qRT-PCR-negative sera reacted with all six viral antigens (100% specificity), and 48% (70/146) were negative throughout the panel. Our findings confirm a low seroprevalence of anti-SARS-CoV-2 antibodies in the Israeli adult population prior to the COVID-19 outbreak. We further suggest that the presence of low-level cross-reacting antibodies in naive individuals calls for a combined, multiantigen analysis for accurate discrimination between naive and exposed individuals. IMPORTANCE A 6-plex protein array presenting the whole inactivated virus and five nucleocapsid and spike-derived SARS-CoV-2 antigens was used to generate a serological snapshot of SARS-CoV-2 seroprevalence and seroconversion in Israel in the early months of the pandemic. Our findings confirm a very low seroprevalence of anti-SARS-CoV-2 antibodies in the Israeli adult population. We further propose that the presence of low-level nonspecific antibodies in naive individuals calls for a combined, multiantigen analysis for accurate discrimination between naive and exposed individuals enabling accurate determination of seroconversion. The developed assay is currently applied to evaluate immune responses to the Israeli vaccine during human phase I/II trials.
Keywords: COVID-19; Israel; SARS-CoV-2; microarrays; multiplex; nucleocapsid; serology; spike.
Figures




References
-
- Oved K, Olmer L, Shemer-Avni Y, Wolf T, Supino-Rosin L, Prajgrod G, Shenhar Y, Payorsky I, Cohen Y, Kohn Y, Indenbaum V, Lazar R, Geylis V, Oikawa MT, Shinar E, Stoyanov E, Keinan-Boker L, Bassal R, Reicher S, Yishai R, Bar-Chaim A, Doolman R, Reiter Y, Mendelson E, Livneh Z, Freedman LS, Lustig Y. 2020. Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 non-responding seronegative subpopulation. EClinicalMedicine 29–30:100651. doi: 10.1016/j.eclinm.2020.100651. - DOI - PMC - PubMed
-
- Jaffe-Hoffman M. 2020. 5.5% of Israelis had coronavirus - serological survey results. Jerusalem Post. https://www.jpost.com/health-science/55-percent-of-israelis-had-coronavi....
-
- Havers FP, Reed C, Lim T, Montgomery JM, Klena JD, Hall AJ, Fry AM, Cannon DL, Chiang CF, Gibbons A, Krapiunaya I, Morales-Betoulle M, Roguski K, Rasheed MAU, Freeman B, Lester S, Mills L, Carroll DS, Owen SM, Johnson JA, Semenova V, Blackmore C, Blog D, Chai SJ, Dunn A, Hand J, Jain S, Lindquist S, Lynfield R, Pritchard S, Sokol T, Sosa L, Turabelidze G, Watkins SM, Wiesman J, Williams RW, Yendell S, Schiffer J, Thornburg NJ. 2020. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med 180:1576–1586. doi: 10.1001/jamainternmed.2020.4130. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

