Methylphenidate Improves Autonomic Functioning among Youth with Attention-Deficit/Hyperactivity Disorder
- PMID: 34613513
- PMCID: PMC8983789
- DOI: 10.1007/s10802-021-00870-5
Methylphenidate Improves Autonomic Functioning among Youth with Attention-Deficit/Hyperactivity Disorder
Abstract
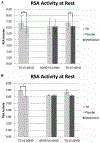
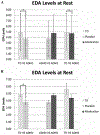
Psychostimulants are commonly prescribed medications for youth with attention-deficit/hyperactivity disorder (ADHD). Limited studies have evaluated how psychostimulants (e.g., methylphenidate [MPH]) impact autonomic nervous system (ANS) indexes among youth with ADHD. Understanding the effects of MPH on autonomic functioning is essential, given that youth with ADHD have been shown to experience atypical autonomic functioning (i.e., reduced activity across both sympathetic and parasympathetic branches) compared to typically developing youth. The current study investigated how a specific psychostimulant, Osmotic Release Oral System [OROS] MPH, impacts parasympathetic (indexed by respiratory sinus arrhythmia [RSA]) and sympathetic (indexed by electrodermal activity [EDA]) functioning among youth with ADHD via a within-subjects, double-masked, cross-over design. Two hundred fifty-six participants (157 youth with ADHD), ages 5 to 13 years, completed a two-minute resting baseline task while electrocardiograph and electrodermal data were obtained. Youth with ADHD completed the resting baseline task twice, 3 weeks apart, once during active medication and once during placebo conditions (counterbalanced). Typically developing youth were assessed without medication or placebo. Youth with ADHD during the placebo condition exhibited reduced RSA and EDA compared to typically developing youth. In contrast, youth with ADHD during the medication condition did not differ significantly from typically developing youth with respect to either RSA nor EDA. As such, OROS MPH appears to normalize RSA and EDA levels among youth with ADHD to levels comparable to typically developing youth. Future studies including indexes of the ANS among youth with ADHD are urged to consider the impact of MPH.
Keywords: Attention-deficit/hyperactivity disorder; Electrodermal activity; Methylphenidate; Respiratory sinus arrhythmia.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Figures
References
-
- American Psychiatric Association. (1994). Diagnostic And Statistical Manual of Mental Disorders : DSM-IV.
-
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
