Mechanisms of Immunomodulation and Cytoprotection Conferred to Pancreatic Islet by Human Amniotic Epithelial Cells
- PMID: 34613550
- PMCID: PMC8799589
- DOI: 10.1007/s12015-021-10269-w
Mechanisms of Immunomodulation and Cytoprotection Conferred to Pancreatic Islet by Human Amniotic Epithelial Cells
Abstract
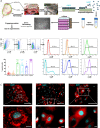
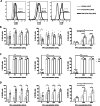
Inhibiting pro-inflammatory cytokine activity can reverse inflammation mediated dysfunction of islet grafts. Human amniotic epithelial cells (hAECs) possess regenerative, immunomodulatory and anti-inflammatory properties. We hypothesized that hAECs could protect islets from cellular damage induced by pro-inflammatory cytokines. To verify our hypothesis, hAEC monocultures, rat islets (RI), or RI-hAEC co-cultures where exposed to a pro-inflammatory cytokine cocktail (Interferon γ: IFN-γ, Tumor necrosis factor α: TNF-α and Interleukin-1β: IL-1β). The secretion of anti-inflammatory cytokines and gene expression changes in hAECs and viability and function of RI were evaluated. The expression of non-classical Major Histocompatibility Complex (MHC) class I molecules by hAECs cultured with various IFN-γ concentrations were assessed. Exposure to the pro-inflammatory cocktail significantly increased the secretion of the anti-inflammatory cytokines IL6, IL10 and G-CSF by hAECs, which was confirmed by upregulation of IL6, and IL10 gene expression. HLA-G, HLA-E and PDL-1 gene expression was also increased. This correlated with an upregulation of STAT1, STAT3 and NF-κB1gene expression levels. RI co-cultured with hAECs maintained normal function after cytokine exposure compared to RI cultured alone, and showed significantly lower apoptosis rate. Our results show that exposure to pro-inflammatory cytokines stimulates secretion of anti-inflammatory and immunomodulatory factors by hAECs through the JAK1/2 - STAT1/3 and the NF-κB1 pathways, which in turn protects islets against inflammation-induced damages. Integrating hAECs in islet transplants appears as a valuable strategy to achieve to inhibit inflammation mediated islet damage, prolong islet survival, improve their engraftment and achieve local immune protection allowing reducing systemic immunosuppressive regimens. This study focuses on the cytoprotective effect of isolated hAECs on islets exposed to pro-inflammatory cytokines in vitro. Exposure to pro-inflammatory cytokines stimulated secretion of anti-inflammatory and immunomodulatory factors by hAECs putatively through the JAK1/2 - STAT1/3 and the NF-κB1 pathways. This had protective effect on islets against inflammation-induced damages. Taken together our results indicate that incorporating hAECs in islet transplants could be a valuable strategy to inhibit inflammation mediated islet damage, prolong islet survival, improve their engraftment and achieve local immune protection allowing to reduce systemic immunosuppressive regimens.
Keywords: Cytoprotection; Human amniotic epithelial cells; Immonomodulation; Pancreatic islets; Pro-inflammatory cytokines.
© 2021. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

References
-
- Carbone, A., Castellani, S., Favia, M., Diana, A., Paracchini, V., Di Gioia, S., … Conese, M. (2014). Correction of defective CFTR/ENaC function and tightness of cystic fibrosis airway epithelium by amniotic mesenchymal stromal (stem) cells. Journal of Cellular and Molecular Medicine,18(8), 1631–1643. 10.1111/jcmm.12303 - PMC - PubMed
-
- Cargnoni, A., Gibelli, L., Tosini, A., Signoroni, P. B., Nassuato, C., Arienti, D., … Parolini, O. (2009). Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transplantation,18(4), 405–422. 10.3727/096368909788809857 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

