Electrophilic Fluorination of Alkenes via Bora-Wagner-Meerwein Rearrangement. Access to β-Difluoroalkyl Boronates
- PMID: 34613633
- PMCID: PMC9299629
- DOI: 10.1002/anie.202109461
Electrophilic Fluorination of Alkenes via Bora-Wagner-Meerwein Rearrangement. Access to β-Difluoroalkyl Boronates
Abstract
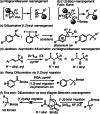
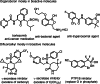
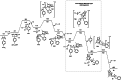
The electrophilic fluorination of geminal alkyl substituted vinyl-Bmida derivatives proceeds via bora-Wagner-Meerwein rearrangement. According to DFT modelling studies this rearrangement occurs with a low activation barrier via a bora-cyclopropane shaped TS. The Bmida group has a larger migration aptitude than the alkyl moiety in the Wagner-Meerwein rearrangement of the presented electrophilic fluorination reactions.
Keywords: DFT modeling; boron; fluorination; organic synthesis; rearrangement.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- None
-
- Hall D. G., Boronic Acids, Wiley-VCH, Weinheim, 2011;
-
- Szabó K. J., Selander N., Organofluorine Chemistry: Synthesis Modeling, and Applications, Wiley-VCH, Weinheim, 2021;
-
- Cahard D., Ma J.-A., Emerging Fluorinated Motifs: Synthesis Properties, and Applications, Wiley-VCH, Weinheim, 2020;
-
- Diner C., Szabó K. J., J. Am. Chem. Soc. 2017, 139, 2–14. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

