Therapeutic targeting of SLC6A8 creatine transporter suppresses colon cancer progression and modulates human creatine levels
- PMID: 34613776
- PMCID: PMC8494442
- DOI: 10.1126/sciadv.abi7511
Therapeutic targeting of SLC6A8 creatine transporter suppresses colon cancer progression and modulates human creatine levels
Abstract
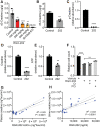
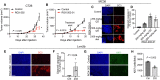
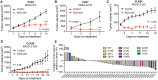
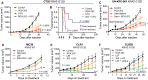
Colorectal cancer (CRC) is a leading cause of cancer mortality. Creatine metabolism was previously shown to critically regulate colon cancer progression. We report that RGX-202, an oral small-molecule SLC6A8 transporter inhibitor, robustly inhibits creatine import in vitro and in vivo, reduces intracellular phosphocreatine and ATP levels, and induces tumor apoptosis. RGX-202 suppressed CRC growth across KRAS wild-type and KRAS mutant xenograft, syngeneic, and patient-derived xenograft (PDX) tumors. Antitumor efficacy correlated with tumoral expression of creatine kinase B. Combining RGX-202 with 5-fluorouracil or the DHODH inhibitor leflunomide caused regressions of multiple colorectal xenograft and PDX tumors of distinct mutational backgrounds. RGX-202 also perturbed creatine metabolism in patients with metastatic CRC in a phase 1 trial, mirroring pharmacodynamic effects on creatine metabolism observed in mice. This is, to our knowledge, the first demonstration of preclinical and human pharmacodynamic activity for creatine metabolism targeting in oncology, thus revealing a critical therapeutic target.
Figures


References
-
- Siegel R. L., Miller K. D., Goding Sauer A., Fedewa S. A., Butterly L. F., Anderson J. C., Cercek A., Smith R. A., Jemal A., Colorectal cancer statistics, 2020. CA Cancer J. Clin. 70, 145–164 (2020). - PubMed
-
- Bridgewater J. A., Pugh S. A., Maishman T., Eminton Z., Mellor J., Whitehead A., Stanton L., Radford M., Corkhill A., Griffiths G. O., Falk S., Valle J. W., O’Reilly D., Siriwardena A. K., Hornbuckle J., Rees M., Iveson T. J., Hickish T., Garden O. J., Cunningham D., Maughan T. S., Primrose J. N.; New EPOC investigators , Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): Long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 21, 398–411 (2020). - PMC - PubMed
-
- Welch S., Spithoff K., Rumble R. B., Maroun J.; Gastrointestinal Cancer Disease Site Group , Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: A systematic review. Ann. Oncol. 21, 1152–1162 (2010). - PubMed
-
- Canon J., Rex K., Saiki A. Y., Mohr C., Cooke K., Bagal D., Gaida K., Holt T., Knutson C. G., Koppada N., Lanman B. A., Werner J., Rapaport A. S., Miguel T. S., Ortiz R., Osgood T., Sun J. R., Zhu X., McCarter J. D., Volak L. P., Houk B. E., Fakih M. G., O’Neil B. H., Price T. J., Falchook G. S., Desai J., Kuo J., Govindan R., Hong D. S., Ouyang W., Henary H., Arvedson T., Cee V. J., Lipford J. R., The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 575, 217–223 (2019). - PubMed
-
- Patricelli M. P., Janes M. R., Li L. S., Hansen R., Peters U., Kessler L. V., Chen Y., Kucharski J. M., Feng J., Ely T., Chen J. H., Firdaus S. J., Babbar A., Ren P., Liu Y., Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov. 6, 316–329 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

