Skeletal muscle regeneration with robotic actuation-mediated clearance of neutrophils
- PMID: 34613813
- PMCID: PMC8961724
- DOI: 10.1126/scitranslmed.abe8868
Skeletal muscle regeneration with robotic actuation-mediated clearance of neutrophils
Abstract
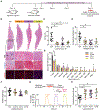
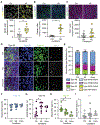
Mechanical stimulation (mechanotherapy) can promote skeletal muscle repair, but a lack of reproducible protocols and mechanistic understanding of the relation between mechanical cues and tissue regeneration limit progress in this field. To address these gaps, we developed a robotic device equipped with real-time force control and compatible with ultrasound imaging for tissue strain analysis. We investigated the hypothesis that specific mechanical loading improves tissue repair by modulating inflammatory responses that regulate skeletal muscle regeneration. We report that cyclic compressive loading within a specific range of forces substantially improves functional recovery of severely injured muscle in mice. This improvement is attributable in part to rapid clearance of neutrophil populations and neutrophil-mediated factors, which otherwise may impede myogenesis. Insights from this work will help advance therapeutic strategies for tissue regeneration broadly.
Conflict of interest statement
Figures
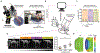


References
-
- Turner NJ, Badylak SF, Regeneration of skeletal muscle. Cell Tissue Res. 347, 759–774 (2012). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

