Development of synthetic antigen vaccines for COVID-19
- PMID: 34613880
- PMCID: PMC8506811
- DOI: 10.1080/21645515.2021.1974288
Development of synthetic antigen vaccines for COVID-19
Abstract
The current pandemic called COVID-19 caused by the SARS-CoV-2 virus brought the need for the search for fast alternatives to both control and fight the SARS-CoV-2 infection. Therefore, a race for a vaccine against COVID-19 took place, and some vaccines have been approved for emergency use in several countries in a record time. Ongoing prophylactic research has sought faster, safer, and precise alternatives by redirecting knowledge of other vaccines, and/or the development of new strategies using available tools, mainly in the areas of genomics and bioinformatics. The current review highlights the development of synthetic antigen vaccines, focusing on the usage of bioinformatics tools for the selection and construction of antigens on the different vaccine constructions under development, as well as strategies to optimize vaccines for COVID-19.
Keywords: SARS-CoV-2; adjuvants; immunoinformatics; in silico; nucleic acid vaccines.
Figures

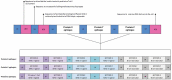

References
-
- World Health Organization (WHO) . Coronavirus disease (COVID-19) dashboard. Geneva, Switzerland: World Health Organization; acessed 2021 Jul 31. https://covid19.who.int/.
-
- DeFrancesco L. Whither COVID-19 vaccines? Nat Biotechnol. 2020. [acessed 2021 Mar 2]. https://www.nature.com/articles/s41587-020-0697-7#citeas. - PMC - PubMed
-
- World Health Organization (WHO) . Global research on coronavírus disease (COVID-19). Geneva, Switzerland: World Health Organization; [acessed 2021 Feb 3]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-r....
-
- Bernasconi V, Kristiansen PA, Whelan M, Román RG, Bettis A, Yimer SA, Gurry C, Andersen SR, Yeskey D, Mandi H, et al. Entwicklung von Impfstoffen gegen neu auftretende Infektionskrankheiten mit epidemischem Potenzial. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2020;63(1):65–73. doi:10.1002/prot.21078. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
