Validation of POD24 as a robust early clinical end point of poor survival in FL from 5225 patients on 13 clinical trials
- PMID: 34614146
- PMCID: PMC9974165
- DOI: 10.1182/blood.2020010263
Validation of POD24 as a robust early clinical end point of poor survival in FL from 5225 patients on 13 clinical trials
Abstract
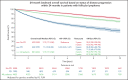
Observational studies and stand-alone trials indicate that patients with follicular lymphoma (FL) who experience disease progression within 24 months of front-line chemoimmunotherapy (POD24), have poor outcomes. We performed a pooled analysis of 13 randomized clinical trials of patients with FL in the pre- and postrituximab eras to identify clinical factors that predict POD24. Logistic regression models evaluated the association between clinical factors and POD24. Cox regression evaluated the association between POD24 as a time-dependent factor and subsequent overall survival (OS). A landmark analysis evaluated the association of POD24 with OS for the subset of patients who were alive at 24 months after trial registration. Patients without progression at 24 months at baseline had favorable performance status (PS), limited-stage (I/II) disease, low-risk FL International Prognostic Index (FLIPI) score, normal baseline hemoglobin, and normal baseline β2 microglobulin (B2M) level. In a multivariable logistic regression model, male sex (odds ratio [OR], 1.30), PS ≥2 (OR, 1.63), B2M (≥3 mg/L; OR, 1.43), and high-risk FLIPI score (3-5; OR, 3.14) were associated with increased risk of progression before 24 months. In the time-dependent Cox model and the 24-month landmark analysis, POD24 was associated with poor subsequent OS (hazard ratio, 4.85 and 3.06, respectively). This is the largest pooled analysis of clinical trials data validating POD24 as a robust indicator of poor FL survival and identified clinical predictors of early death and progression that can aid in building comprehensive prognostic models incorporating clinical and molecular predictors of POD24.
© 2022 by The American Society of Hematology.
Figures


Comment in
-
POD24 in follicular lymphoma: time to be "wise".Blood. 2022 Mar 17;139(11):1609-1610. doi: 10.1182/blood.2021013437. Blood. 2022. PMID: 35298602 No abstract available.
References
-
- Casulo C, Burack WR, Friedberg JW. Transformed follicular non-Hodgkin lymphoma. Blood. 2015;125(1):40–47. - PubMed
-
- Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study [published correction appears in J Clin Oncol. 2016;34(12):1430] J Clin Oncol. 2015;33(23):2516–2522. - PMC - PubMed
-
- Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377(9759):42–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

