Immunological non-inferiority of a new fully liquid presentation of the MenACWY-CRM vaccine to the licensed vaccine: results from a randomized, controlled, observer-blind study in adolescents and young adults
- PMID: 34614379
- PMCID: PMC8966988
- DOI: 10.1080/21645515.2021.1981085
Immunological non-inferiority of a new fully liquid presentation of the MenACWY-CRM vaccine to the licensed vaccine: results from a randomized, controlled, observer-blind study in adolescents and young adults
Abstract
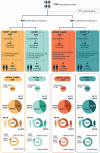
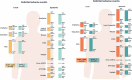
A fully liquid MenACWY-CRM vaccine presentation has been developed, modifying the meningococcal serogroup A (MenA) component from lyophilized to liquid. The safety and immunogenicity of the liquid presentation at the end of the intended shelf-life (aged for 24 or 30 months) were compared to the licensed lyophilized/liquid presentation. This multicenter, randomized (1:1), observer-blind, phase 2b study (NCT03433482) enrolled adolescents and young adults (age 10-40 years). In part 1, 844 participants received one dose of liquid presentation stored for approximately 24 months or licensed presentation. In part 2, 846 participants received one dose of liquid presentation stored for approximately 30 months or licensed presentation. After storage, the MenA free saccharide (FS) level was approximately 25% and O-acetylation was approximately 45%. The primary objective was to demonstrate non-inferiority of the liquid presentation to licensed presentation, as measured by human serum bactericidal assay (hSBA) geometric mean titers (GMTs) against MenA, 1-month post-vaccination. Immune responses against each vaccine serogroup were similar between groups. Between-group ratios of hSBA GMTs for MenA were 1.21 (part 1) and 1.11 (part 2), with two-sided 95% confidence interval lower limits (0.94 and 0.87, respectively) greater than the prespecified non-inferiority margin (0.5), thus meeting the primary study objective. No safety concerns were identified. Despite reduced O-acetylation of MenA and increased FS content, serogroup-specific immune responses induced by the fully liquid presentation were similar to those induced by the licensed MenACWY-CRM vaccine, with non-inferior anti-MenA responses. The safety profiles of the vaccine presentations were similar.
Keywords: MenACWY-CRM; adolescents; human serum bactericidal assay; meningococcal serogroup A; non-inferiority.
Conflict of interest statement
T.A., V.R., M.C., T.M., B.K., I.L., M.L., E.F., V.B., M.A., J.-A.G.-M., P.T., K.N., and M.P. are employed by the GSK group of companies. T.A., M.L., E.F., V.B., M.A., and M.P. hold shares in the GSK group of companies. J.D.-D. reports payments from the GSK group of companies and Sanofi Pasteur to his institution, outside the submitted work. E.C.D. reports payments from the GSK group of companies to his institution for the conduct of the study, and Sanofi Pasteur and Pfizer, outside the submitted work. I.S.C. reports payments from the GSK group of companies, Sanofi Pasteur, MSD, and Pfizer, outside the submitted work. E.D.M. Jr reports payments from the GSK group of companies to his institution for the conduct of the study. J.D.-D., E.C.D., I.S.C., E.D.M. Jr, T.A., V.R., M.C., T.M., B.K., I.L., M.L., E.F., V.B., M.A., J.-A.G.-M., P.T., K.N., and M.P. declare no other financial and non-financial relationships and activities. J.C.T., A.P., H.N., I.S.C., T.I., K.A., K.L., Y.K. and C.E.M.P. declare no financial and non-financial relationships and activities and no conflicts of interest.
Figures



References
-
- Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, Christensen H, Climent Y, De Wals P, Dinleyici EC, et al. The global meningococcal initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines. 2019;18(1):15–9. doi:10.1080/14760584.2019.1557520. - DOI - PubMed
-
- Parikh S, Campbell H, Bettinger JA, Harrison LH, Marshall HS, Martinon-Torres F, Safadi MA, Shao Z, Zhu B, von Gottberg A, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81:483–98. doi:10.1016/j.jinf.2020.05.079. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
