Spillover, hybridization, and persistence in schistosome transmission dynamics at the human-animal interface
- PMID: 34615712
- PMCID: PMC8521685
- DOI: 10.1073/pnas.2110711118
Spillover, hybridization, and persistence in schistosome transmission dynamics at the human-animal interface
Abstract
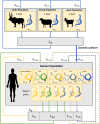
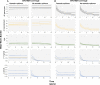
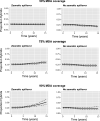
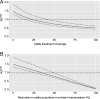
Zoonotic spillover and hybridization of parasites are major emerging public and veterinary health concerns at the interface of infectious disease biology, evolution, and control. Schistosomiasis is a neglected tropical disease of global importance caused by parasites of the Schistosoma genus, and the Schistosoma spp. system within Africa represents a key example of a system where spillover of animal parasites into human populations has enabled formation of hybrids. Combining model-based approaches and analyses of parasitological, molecular, and epidemiological data from northern Senegal, a region with a high prevalence of schistosome hybrids, we aimed to unravel the transmission dynamics of this complex multihost, multiparasite system. Using Bayesian methods and by estimating the basic reproduction number (R0 ), we evaluate the frequency of zoonotic spillover of Schistosoma bovis from livestock and the potential for onward transmission of hybrid S. bovis × S. haematobium offspring within human populations. We estimate R0 of hybrid schistosomes to be greater than the critical threshold of one (1.76; 95% CI 1.59 to 1.99), demonstrating the potential for hybridization to facilitate spread and establishment of schistosomiasis beyond its original geographical boundaries. We estimate R0 for S. bovis to be greater than one in cattle (1.43; 95% CI 1.24 to 1.85) but not in other ruminants, confirming cattle as the primary zoonotic reservoir. Through longitudinal simulations, we also show that where S. bovis and S. haematobium are coendemic (in livestock and humans respectively), the relative importance of zoonotic transmission is predicted to increase as the disease in humans nears elimination.
Keywords: R0; hybrids; modelling; schistosomiasis; spillover.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Aström C., et al., Potential distribution of dengue fever under scenarios of climate change and economic development. EcoHealth 9, 448–454 (2012). - PubMed
-
- Bett B., et al., Effects of climate change on the occurrence and distribution of livestock diseases. Prev. Vet. Med. 137 (pt B), 119–129 (2017). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

