Structural basis of rotavirus RNA chaperone displacement and RNA annealing
- PMID: 34615715
- PMCID: PMC8521686
- DOI: 10.1073/pnas.2100198118
Structural basis of rotavirus RNA chaperone displacement and RNA annealing
Abstract
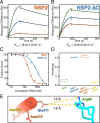
Rotavirus genomes are distributed between 11 distinct RNA molecules, all of which must be selectively copackaged during virus assembly. This likely occurs through sequence-specific RNA interactions facilitated by the RNA chaperone NSP2. Here, we report that NSP2 autoregulates its chaperone activity through its C-terminal region (CTR) that promotes RNA-RNA interactions by limiting its helix-unwinding activity. Unexpectedly, structural proteomics data revealed that the CTR does not directly interact with RNA, while accelerating RNA release from NSP2. Cryo-electron microscopy reconstructions of an NSP2-RNA complex reveal a highly conserved acidic patch on the CTR, which is poised toward the bound RNA. Virus replication was abrogated by charge-disrupting mutations within the acidic patch but completely restored by charge-preserving mutations. Mechanistic similarities between NSP2 and the unrelated bacterial RNA chaperone Hfq suggest that accelerating RNA dissociation while promoting intermolecular RNA interactions may be a widespread strategy of RNA chaperone recycling.
Keywords: RNA chaperones; genome assembly; ribonucleoproteins; rotavirus.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
- 213437/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- BB/M012573/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 220628/WT_/Wellcome Trust/United Kingdom
- 108466/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- BB/M011151/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

