Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 lineages circulating in Brazil
- PMID: 34615860
- PMCID: PMC8494913
- DOI: 10.1038/s41467-021-25982-w
Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 lineages circulating in Brazil
Abstract
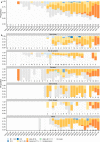
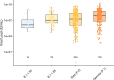
Several COVID-19 vaccines have shown good efficacy in clinical trials, but there remains uncertainty about the efficacy of vaccines against different variants. Here, we investigate the efficacy of ChAdOx1 nCoV-19 (AZD1222) against symptomatic COVID-19 in a post-hoc exploratory analysis of a Phase 3 randomised trial in Brazil (trial registration ISRCTN89951424). Nose and throat swabs were tested by PCR in symptomatic participants. Sequencing and genotyping of swabs were performed to determine the lineages of SARS-CoV-2 circulating during the study. Protection against any symptomatic COVID-19 caused by the Zeta (P.2) variant was assessed in 153 cases with vaccine efficacy (VE) of 69% (95% CI 55, 78). 49 cases of B.1.1.28 occurred and VE was 73% (46, 86). The Gamma (P.1) variant arose later in the trial and fewer cases (N = 18) were available for analysis. VE was 64% (-2, 87). ChAdOx1 nCoV-19 provided 95% protection (95% CI 61%, 99%) against hospitalisation due to COVID-19. In summary, we report that ChAdOx1 nCoV-19 protects against emerging variants in Brazil despite the presence of the spike protein mutation E484K.
© 2021. The Author(s).
Conflict of interest statement
Oxford University has entered into a partnership with AstraZeneca for further development of ChAdOx1 nCoV-19. AstraZeneca reviewed the data from the study and the final manuscript before submission, but the authors retained editorial control. S.C.G. is cofounder of Vaccitech (collaborators in the early development of this vaccine candidate) and named as an inventor on a patent covering use of ChAdOx1-vectored vaccines (PCT/GB2012/000467) and a patent application covering this SARS-CoV-2 vaccine. P.M.F. is a consultant to Vaccitech. A.J.P. is Chair of the UK Department of Health and Social Care’s JCVI, but does not participate in policy advice on coronavirus vaccines, and is a member of the WHO Strategic Advisory Group of Experts (SAGE). A.J.P. is a NIHR Senior Investigator. All other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

