Eliapixant is a selective P2X3 receptor antagonist for the treatment of disorders associated with hypersensitive nerve fibers
- PMID: 34615939
- PMCID: PMC8494816
- DOI: 10.1038/s41598-021-99177-0
Eliapixant is a selective P2X3 receptor antagonist for the treatment of disorders associated with hypersensitive nerve fibers
Abstract
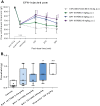
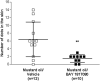
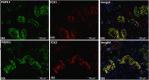
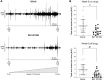
ATP-dependent P2X3 receptors play a crucial role in the sensitization of nerve fibers and pathological pain pathways. They are also involved in pathways triggering cough and may contribute to the pathophysiology of endometriosis and overactive bladder. However, despite the strong therapeutic rationale for targeting P2X3 receptors, preliminary antagonists have been hampered by off-target effects, including severe taste disturbances associated with blocking the P2X2/3 receptor heterotrimer. Here we present a P2X3 receptor antagonist, eliapixant (BAY 1817080), which is both highly potent and selective for P2X3 over other P2X subtypes in vitro, including P2X2/3. We show that eliapixant reduces inflammatory pain in relevant animal models. We also provide the first in vivo experimental evidence that P2X3 antagonism reduces neurogenic inflammation, a phenomenon hypothesised to contribute to several diseases, including endometriosis. To test whether eliapixant could help treat endometriosis, we confirmed P2X3 expression on nerve fibers innervating human endometriotic lesions. We then demonstrate that eliapixant reduces vaginal hyperalgesia in an animal model of endometriosis-associated dyspareunia, even beyond treatment cessation. Our findings indicate that P2X3 antagonism could alleviate pain, including non-menstrual pelvic pain, and modify the underlying disease pathophysiology in women with endometriosis. Eliapixant is currently under clinical development for the treatment of disorders associated with hypersensitive nerve fibers.
© 2021. The Author(s).
Conflict of interest statement
A.J.D., I.N., F.M., A-M.C., S.B., N.C., M.J.G., and S.D.H. are employees of Evotec. N.B., M.K., A.R., J.N., A.L-B., T.M.Z., and O.M.F. are employees of Bayer. Bayer holds a patent on eliapixant (BAY 1817080). A.J.D., I.N., J.N., N.B., O.M.F., A.R., and A.M.C. are inventors on this patent application.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

