Spatially resolved cell atlas of the mouse primary motor cortex by MERFISH
- PMID: 34616063
- PMCID: PMC8494645
- DOI: 10.1038/s41586-021-03705-x
Spatially resolved cell atlas of the mouse primary motor cortex by MERFISH
Abstract
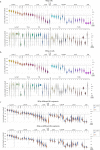
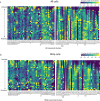
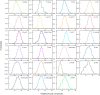
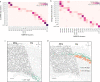
A mammalian brain is composed of numerous cell types organized in an intricate manner to form functional neural circuits. Single-cell RNA sequencing allows systematic identification of cell types based on their gene expression profiles and has revealed many distinct cell populations in the brain1,2. Single-cell epigenomic profiling3,4 further provides information on gene-regulatory signatures of different cell types. Understanding how different cell types contribute to brain function, however, requires knowledge of their spatial organization and connectivity, which is not preserved in sequencing-based methods that involve cell dissociation. Here we used a single-cell transcriptome-imaging method, multiplexed error-robust fluorescence in situ hybridization (MERFISH)5, to generate a molecularly defined and spatially resolved cell atlas of the mouse primary motor cortex. We profiled approximately 300,000 cells in the mouse primary motor cortex and its adjacent areas, identified 95 neuronal and non-neuronal cell clusters, and revealed a complex spatial map in which not only excitatory but also most inhibitory neuronal clusters adopted laminar organizations. Intratelencephalic neurons formed a largely continuous gradient along the cortical depth axis, in which the gene expression of individual cells correlated with their cortical depths. Furthermore, we integrated MERFISH with retrograde labelling to probe projection targets of neurons of the mouse primary motor cortex and found that their cortical projections formed a complex network in which individual neuronal clusters project to multiple target regions and individual target regions receive inputs from multiple neuronal clusters.
© 2021. The Author(s).
Conflict of interest statement
X.Z. is a co-founder and consultant of Vizgen.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

