Human neocortical expansion involves glutamatergic neuron diversification
- PMID: 34616067
- PMCID: PMC8494638
- DOI: 10.1038/s41586-021-03813-8
Human neocortical expansion involves glutamatergic neuron diversification
Erratum in
-
Author Correction: Human neocortical expansion involves glutamatergic neuron diversification.Nature. 2022 Jan;601(7893):E12. doi: 10.1038/s41586-021-04322-4. Nature. 2022. PMID: 34992294 Free PMC article. No abstract available.
Abstract
The neocortex is disproportionately expanded in human compared with mouse1,2, both in its total volume relative to subcortical structures and in the proportion occupied by supragranular layers composed of neurons that selectively make connections within the neocortex and with other telencephalic structures. Single-cell transcriptomic analyses of human and mouse neocortex show an increased diversity of glutamatergic neuron types in supragranular layers in human neocortex and pronounced gradients as a function of cortical depth3. Here, to probe the functional and anatomical correlates of this transcriptomic diversity, we developed a robust platform combining patch clamp recording, biocytin staining and single-cell RNA-sequencing (Patch-seq) to examine neurosurgically resected human tissues. We demonstrate a strong correspondence between morphological, physiological and transcriptomic phenotypes of five human glutamatergic supragranular neuron types. These were enriched in but not restricted to layers, with one type varying continuously in all phenotypes across layers 2 and 3. The deep portion of layer 3 contained highly distinctive cell types, two of which express a neurofilament protein that labels long-range projection neurons in primates that are selectively depleted in Alzheimer's disease4,5. Together, these results demonstrate the explanatory power of transcriptomic cell-type classification, provide a structural underpinning for increased complexity of cortical function in humans, and implicate discrete transcriptomic neuron types as selectively vulnerable in disease.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




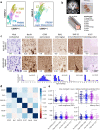


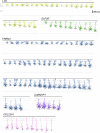


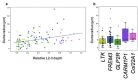

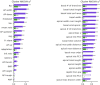
References
-
- Petersen CCH. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. - PubMed
-
- DeFelipe J, Alonso-Nanclares L, Arellano JI. Microstructure of the neocortex: comparative aspects. J. Neurocytol. 2002;31:299–316. - PubMed
-
- Bussière T, et al. Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer’s disease: stereologic analysis of prefrontal cortex area 9. J. Comp. Neurol. 2003;463:281–302. - PubMed
-
- Hof PR, Cox K, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer’s disease: I. Superior frontal and inferior temporal cortex. J. Comp. Neurol. 1990;301:44–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

