Morphological diversity of single neurons in molecularly defined cell types
- PMID: 34616072
- PMCID: PMC8494643
- DOI: 10.1038/s41586-021-03941-1
Morphological diversity of single neurons in molecularly defined cell types
Abstract
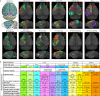
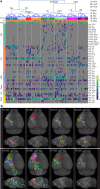
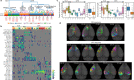
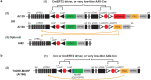
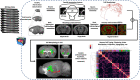
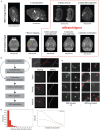
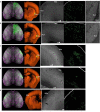
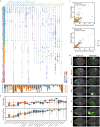
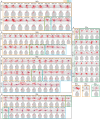
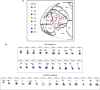
Dendritic and axonal morphology reflects the input and output of neurons and is a defining feature of neuronal types1,2, yet our knowledge of its diversity remains limited. Here, to systematically examine complete single-neuron morphologies on a brain-wide scale, we established a pipeline encompassing sparse labelling, whole-brain imaging, reconstruction, registration and analysis. We fully reconstructed 1,741 neurons from cortex, claustrum, thalamus, striatum and other brain regions in mice. We identified 11 major projection neuron types with distinct morphological features and corresponding transcriptomic identities. Extensive projectional diversity was found within each of these major types, on the basis of which some types were clustered into more refined subtypes. This diversity follows a set of generalizable principles that govern long-range axonal projections at different levels, including molecular correspondence, divergent or convergent projection, axon termination pattern, regional specificity, topography, and individual cell variability. Although clear concordance with transcriptomic profiles is evident at the level of major projection type, fine-grained morphological diversity often does not readily correlate with transcriptomic subtypes derived from unsupervised clustering, highlighting the need for single-cell cross-modality studies. Overall, our study demonstrates the crucial need for quantitative description of complete single-cell anatomy in cell-type classification, as single-cell morphological diversity reveals a plethora of ways in which different cell types and their individual members may contribute to the configuration and function of their respective circuits.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

