A multimodal cell census and atlas of the mammalian primary motor cortex
- PMID: 34616075
- PMCID: PMC8494634
- DOI: 10.1038/s41586-021-03950-0
A multimodal cell census and atlas of the mammalian primary motor cortex
Abstract
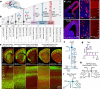
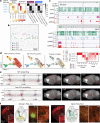
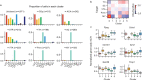
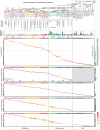
Here we report the generation of a multimodal cell census and atlas of the mammalian primary motor cortex as the initial product of the BRAIN Initiative Cell Census Network (BICCN). This was achieved by coordinated large-scale analyses of single-cell transcriptomes, chromatin accessibility, DNA methylomes, spatially resolved single-cell transcriptomes, morphological and electrophysiological properties and cellular resolution input-output mapping, integrated through cross-modal computational analysis. Our results advance the collective knowledge and understanding of brain cell-type organization1-5. First, our study reveals a unified molecular genetic landscape of cortical cell types that integrates their transcriptome, open chromatin and DNA methylation maps. Second, cross-species analysis achieves a consensus taxonomy of transcriptomic types and their hierarchical organization that is conserved from mouse to marmoset and human. Third, in situ single-cell transcriptomics provides a spatially resolved cell-type atlas of the motor cortex. Fourth, cross-modal analysis provides compelling evidence for the transcriptomic, epigenomic and gene regulatory basis of neuronal phenotypes such as their physiological and anatomical properties, demonstrating the biological validity and genomic underpinning of neuron types. We further present an extensive genetic toolset for targeting glutamatergic neuron types towards linking their molecular and developmental identity to their circuit function. Together, our results establish a unifying and mechanistic framework of neuronal cell-type organization that integrates multi-layered molecular genetic and spatial information with multi-faceted phenotypic properties.
© 2021. The Author(s).
Conflict of interest statement
A. Bandrowski is a cofounder of SciCrunch, a company devoted to improving scientific communication. J.R.E. is a member of Zymo Research SAB. J.A.H., K.E.H., T.N.N. and P.R.N. are currently employed by Cajal Neuroscience. P.V.K. serves on the Scientific Advisory Board of Celsius Therapeutics Inc. M.E.M. is a founder and CSO of SciCrunch Inc., a UCSD tech start up that produces tools in support of reproducibility including RRIDs. A.R. is a founder and equity holder of Celsius Therapeutics, an equity holder in Immunitas Therapeutics and until 31 August 2020 was a member of the scientific advisory board of Syros Pharmaceuticals, Neogene Therapeutics, Asimov and ThermoFisher Scientific. From 1 August 2020, A.R. has been an employee of Genentech. B.R. is a co-founder of Arima Genomics, Inc. and Epigenome Technologies, Inc. K.Z. is a co-founder, equity holder and serves on the Scientific Advisor Board of Singlera Genomics. X. Zhuang is a co-founder and consultant of Vizgen.
Figures











Comment in
-
Collaboration produces brain cell census.Nat Rev Neurosci. 2022 Jan;23(1):1. doi: 10.1038/s41583-021-00541-w. Nat Rev Neurosci. 2022. PMID: 34754097 No abstract available.
References
-
- Sanes, J. R. & Masland, R. H. The types of retinal ganglion cells: current status and implications for neuronal classification. Annu. Rev. Neurosci.38, 221–246 (2015). - PubMed
-
- Zeng, H. & Sanes, J. R. Neuronal cell-type classification: challenges, opportunities and the path forward. Nat. Rev. Neurosci.18, 530–546 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 MH114821/MH/NIMH NIH HHS/United States
- R24 MH114815/MH/NIMH NIH HHS/United States
- U01 MH117072/MH/NIMH NIH HHS/United States
- U01 MH117079/MH/NIMH NIH HHS/United States
- R01 EY023173/EY/NEI NIH HHS/United States
- R33 NS115183/NS/NINDS NIH HHS/United States
- U01 MH121260/MH/NIMH NIH HHS/United States
- U01 MH114828/MH/NIMH NIH HHS/United States
- R01 NS086082/NS/NINDS NIH HHS/United States
- R01 MH094360/MH/NIMH NIH HHS/United States
- U01 MH114829/MH/NIMH NIH HHS/United States
- U01 MH114824/MH/NIMH NIH HHS/United States
- U01 MH114812/MH/NIMH NIH HHS/United States
- P51 OD010425/OD/NIH HHS/United States
- R01 MH120404/MH/NIMH NIH HHS/United States
- R24 MH114793/MH/NIMH NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- U01 MH114825/MH/NIMH NIH HHS/United States
- R24 AG073205/AG/NIA NIH HHS/United States
- P30 AG066509/AG/NIA NIH HHS/United States
- RF1 MH123199/MH/NIMH NIH HHS/United States
- S10 OD023689/OD/NIH HHS/United States
- U01 MH114819/MH/NIMH NIH HHS/United States
- OD010425/NH/NIH HHS/United States
- U01 MH105982/MH/NIMH NIH HHS/United States
- R01 DC019370/DC/NIDCD NIH HHS/United States
- U24 MH114827/MH/NIMH NIH HHS/United States
- R24 MH117295/MH/NIMH NIH HHS/United States
- R01 MH122169/MH/NIMH NIH HHS/United States
- U01 MH121282/MH/NIMH NIH HHS/United States
- R61 NS115183/NS/NINDS NIH HHS/United States
- U19 MH114831/MH/NIMH NIH HHS/United States
- U01 MH117023/MH/NIMH NIH HHS/United States
- UL1 TR000423/TR/NCATS NIH HHS/United States
- R24 MH114788/MH/NIMH NIH HHS/United States
- R01 NS039600/NS/NINDS NIH HHS/United States
- R01 NS110767/NS/NINDS NIH HHS/United States
- U19 MH114830/MH/NIMH NIH HHS/United States
- RF1 MH114126/MH/NIMH NIH HHS/United States
- U01 MH116990/MH/NIMH NIH HHS/United States
- P30 CA014195/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

