Real-World Experience of Bevacizumab as First-Line Treatment for Ovarian Cancer: The GINECO ENCOURAGE Cohort of 468 French Patients
- PMID: 34616296
- PMCID: PMC8489574
- DOI: 10.3389/fphar.2021.711813
Real-World Experience of Bevacizumab as First-Line Treatment for Ovarian Cancer: The GINECO ENCOURAGE Cohort of 468 French Patients
Abstract
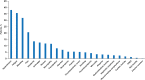
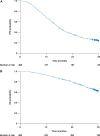
Introduction: Bevacizumab-containing therapy is considered a standard-of-care front-line option for stage IIIB-IV ovarian cancer based on results of randomized phase 3 trials. The multicenter non-interventional ENCOURAGE prospective cohort study assessed treatment administration and outcomes in the French real-world setting. Patients and Methods: Eligible patients were aged ≥ 18 years with planned bevacizumab-containing therapy for newly diagnosed ovarian cancer. The primary objective was to assess the safety profile of front-line bevacizumab in routine clinical practice; secondary objectives were to describe patient characteristics, indications/contraindications for bevacizumab, treatment regimens and co-medications, follow-up and monitoring, progression-free survival, and treatment at recurrence. In this non-interventional study, treatment was administered as chosen by the investigator and participation in the trial had no influence on the management of the disease. Results: Of 1,290 patients screened between April 2013 and February 2015, 468 were eligible. Most patients (86%) received bevacizumab 15 mg/kg every 3 weeks or equivalent, typically with carboplatin (99%) and paclitaxel (98%). The median duration of bevacizumab was 12.2 (range 0-28, interquartile range 6.9-14.9) months; 8% of patients discontinued bevacizumab because of toxicity. The most common adverse events were hypertension (38% of patients), fatigue (35%), and bleeding (32%). There were no treatment-related deaths. Most physicians (90%) reported blood pressure measurement immediately before each bevacizumab infusion and almost all (97%) reported monitoring for proteinuria before each bevacizumab infusion. Median progression-free survival was 17.4 (95% CI, 16.4-19.1) months. The 3-year overall survival rate was 62% (95% CI, 58-67%). The most commonly administered chemotherapies at recurrence were carboplatin and pegylated liposomal doxorubicin. Discussion: Clinical outcomes and tolerability with bevacizumab in this real-life setting are consistent with randomized trial results, notwithstanding differences in the treated patient population and treatment schedule. Clinical Trial Registration:ClinicalTrials.gov, Identifier NCT01832415.
Keywords: bevacizumab; monitoring; ovarian cancer; progression-free survival; routine clinical practice.
Copyright © 2021 Berton, Floquet, Lescaut, Baron, Kaminsky, Toussaint, Largillier, Savoye, Alexandre, Delbaldo, Malaurie, Barletta, Bosacki, Garnier-Tixidre, Follana, Laharie-Mineur, Briac Levache, Valenza, Dechartres, Mollon-Grange and Selle.
Conflict of interest statement
AF reports honoraria and travel/accommodation/expenses from AstraZeneca, Clovis, and GSK, and non-remunerated activities for MSD and Roche. PT reports honoraria from MSD, Tesaro/GSK, and Pfizer, and advisory/consultancy roles for MSD and Tesaro/GSK. JA reports honoraria from GSK, AstraZeneca, MSD, PharmaMar, and Eisai; travel/accommodation/expenses from Novartis, Janssen, and GSK; and research grant/funding to his institution from Janssen and MSD. CG-T reports honoraria from and advisory/consultancy roles for Pfizer; speaker bureau/expert testimony for AstraZeneca and MSD; travel/accommodation/expenses from MSD, Pfizer, and Mylan; and research grant/funding to her institution from Roche. PF reports honoraria from and advisory/consultancy roles for Novartis, AstraZeneca, GSK, and Clovis, and travel/accommodation/expenses from Novartis, AstraZeneca, and GSK. CBL reports honoraria (paid to his institution) from Roche, Novartis, Isofol, Array Biopharma, AstraZeneca, BMS, and Celgene. FS reports honoraria from Roche, AstraZeneca, Tesaro, Clovis, MSD, PharmaMar, and GlaxoSmithKline; advisory/consultancy role for Roche; speaker bureau/expert testimony for Roche, AstraZeneca, Tesaro, and GlaxoSmithKline; and travel/accommodation/expenses from Roche, AstraZeneca, Tesaro, MSD, and PharmaMar. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Avastin Prescribing Information (2020). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125085s332lbl.pdf (Accessed Mar 24, 2021).
-
- de la Motte Rouge T., Ray-Coquard I., You B. (2019). [Medical Treatment in Ovarian Cancers Newly Diagnosed: Article Drafted from the French Guidelines in Oncology Entitled "Initial Management of Patients with Epithelial Ovarian Cancer" Developed by FRANCOGYN, CNGOF, SFOG, GINECO-ARCAGY under the Aegis of CNGOF and Endorsed by INCa]. Gynecol. Obstet. Fertil. Senol 47, 222–237. 10.1016/j.gofs.2019.01.002 - DOI - PubMed
-
- Institut National du Cancer (INCa) (2021). Tumeurs solides - Estimations nationales de l’incidence et de la mortalité par cancer en France métropolitaine entre 1990 et 2018. Available at: https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publica... (Accessed Mar 24, 2021).
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

