SARS-CoV-2 Coronavirus Spike Protein-Induced Apoptosis, Inflammatory, and Oxidative Stress Responses in THP-1-Like-Macrophages: Potential Role of Angiotensin-Converting Enzyme Inhibitor (Perindopril)
- PMID: 34616396
- PMCID: PMC8488399
- DOI: 10.3389/fimmu.2021.728896
SARS-CoV-2 Coronavirus Spike Protein-Induced Apoptosis, Inflammatory, and Oxidative Stress Responses in THP-1-Like-Macrophages: Potential Role of Angiotensin-Converting Enzyme Inhibitor (Perindopril)
Abstract
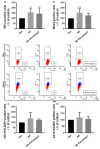
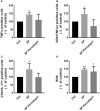
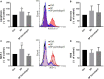
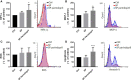
A purified spike (S) glycoprotein of severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) coronavirus was used to study its effects on THP-1 macrophages, peripheral blood mononuclear cells (PBMCs), and HUVEC cells. The S protein mediates the entry of SARS-CoV-2 into cells through binding to the angiotensin-converting enzyme 2 (ACE2) receptors. We measured the viability, intracellular cytokine release, oxidative stress, proinflammatory markers, and THP-1-like macrophage polarization. We observed an increase in apoptosis, ROS generation, MCP-1, and intracellular calcium expression in the THP-1 macrophages. Stimulation with the S protein polarizes the THP-1 macrophages towards proinflammatory futures with an increase in the TNFα and MHC-II M1-like phenotype markers. Treating the cells with an ACE inhibitor, perindopril, at 100 µM reduced apoptosis, ROS, and MHC-II expression induced by S protein. We analyzed the sensitivity of the HUVEC cells after the exposure to a conditioned media (CM) of THP-1 macrophages stimulated with the S protein. The CM induced endothelial cell apoptosis and MCP-1 expression. Treatment with perindopril reduced these effects. However, the direct stimulation of the HUVEC cells with the S protein, slightly increased HIF1α and MCP-1 expression, which was significantly increased by the ACE inhibitor treatment. The S protein stimulation induced ROS generation and changed the mitogenic responses of the PBMCs through the upregulation of TNFα and interleukin (IL)-17 cytokine expression. These effects were reduced by the perindopril (100 µM) treatment. Proteomic analysis of the S protein stimulated THP-1 macrophages with or without perindopril (100 µM) exposed more than 400 differentially regulated proteins. Our results provide a mechanistic analysis suggesting that the blood and vascular components could be activated directly through S protein systemically present in the circulation and that the activation of the local renin angiotensin system may be partially involved in this process.
Graphical: Suggested pathways that might be involved at least in part in S protein inducing activation of inflammatory markers (red narrow) and angiotensin-converting enzyme inhibitor (ACEi) modulation of this process (green narrow).
Keywords: HUVEC cells; ROS; SARS-CoV-2; angiotensin-converting enzyme inhibitor; inflammation; monocyte/macrophages; spike protein.
Copyright © 2021 Barhoumi, Alghanem, Shaibah, Mansour, Alamri, Akiel, Alroqi and Boudjelal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

