Liposomal formulations of anticancer copper(II) thiosemicarbazone complexes
- PMID: 34617075
- PMCID: PMC8594434
- DOI: 10.1039/d1dt02763h
Liposomal formulations of anticancer copper(II) thiosemicarbazone complexes
Abstract
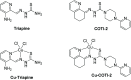
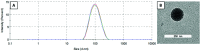
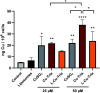
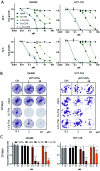
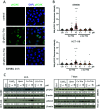
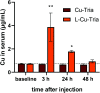
α-N-Heterocyclic thiosemicarbazones such as triapine and COTI-2 are currently investigated as anticancer therapeutics in clinical trials. However, triapine was widely inactive against solid tumor types. A likely explanation is the short plasma half-life time and fast metabolism. One promising approach to overcome these drawbacks is the encapsulation of the drug into nanoparticles (passive drug-targeting). In a previous work we showed that it was not possible to stably encapsulate free triapine into liposomes. Hence, in this manuscript we present the successful preparation of liposomal formulations of the copper(II) complexes of triapine and COTI-2. To this end, various drug-loading strategies were examined and the resulting liposomes were physico-chemically characterized. Especially for liposomal Cu-triapine, a decent encapsulation efficacy and a slow drug release behavior could be observed. In contrast, for COTI-2 and its copper(II) complex no stable loading could be achieved. Subsequent in vitro studies in different cell lines with liposomal Cu-triapine showed the expected strongly reduced cytotoxicity and DNA damage induction. Also in vivo distinctly higher copper plasma levels and a continuous release could be observed for the liposomal formulation compared to free Cu-triapine. Taken together, the here presented nanoformulation of Cu-triapine is an important step further to increase the plasma half-life time and tumor targeting properties of anticancer thiosemicarbazones.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Lobana T. S. Sharma R. Bawa G. Khanna S. Bonding and structure trends of thiosemicarbazone derivatives of metals—an overview. Coord. Chem. Rev. 2009;253(7–8):977–1055. doi: 10.1016/j.ccr.2008.07.004. - DOI
-
- Yu Y. Kalinowski D. S. Kovacevic Z. Siafakas A. R. Jansson P. J. Stefani C. Lovejoy D. B. Sharpe P. C. Bernhardt P. V. Richardson D. R. Thiosemicarbazones from the old to new: iron chelators that are more than just ribonucleotide reductase inhibitors. J. Med. Chem. 2009;52(17):5271–5294. doi: 10.1021/jm900552r. - DOI - PubMed
-
- Hager S. Pape V. F. Pósa V. Montsch B. Uhlik L. Szakács G. Tóth S. Jabronka N. Keppler B. K. Kowol C. R. High copper complex stability and slow reduction kinetics as key parameters for improved activity, paraptosis induction and impact on drug-resistant cells of anticancer thiosemicarbazones. Antioxid. Redox Signal. 2020;33(6):395–414. doi: 10.1089/ars.2019.7854. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

