Ibrutinib Plus Venetoclax for First-Line Treatment of Chronic Lymphocytic Leukemia: Primary Analysis Results From the Minimal Residual Disease Cohort of the Randomized Phase II CAPTIVATE Study
- PMID: 34618601
- PMCID: PMC8713593
- DOI: 10.1200/JCO.21.00807
Ibrutinib Plus Venetoclax for First-Line Treatment of Chronic Lymphocytic Leukemia: Primary Analysis Results From the Minimal Residual Disease Cohort of the Randomized Phase II CAPTIVATE Study
Abstract
Purpose: CAPTIVATE (NCT02910583), a randomized phase II study, evaluates minimal residual disease (MRD)-guided treatment discontinuation following completion of first-line ibrutinib plus venetoclax treatment in patients with chronic lymphocytic leukemia (CLL).
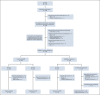
Methods: Previously untreated CLL patients age < 70 years received three cycles of ibrutinib and then 12 cycles of combined ibrutinib plus venetoclax. Patients in the MRD cohort who met the stringent random assignment criteria for confirmed undetectable MRD (Confirmed uMRD) were randomly assigned 1:1 to double-blind placebo or ibrutinib; patients without Confirmed uMRD (uMRD Not Confirmed) were randomly assigned 1:1 to open-label ibrutinib or ibrutinib plus venetoclax. Primary end point was 1-year disease-free survival (DFS) rate with placebo versus ibrutinib in the Confirmed uMRD population. Secondary end points included response rates, uMRD, and safety.
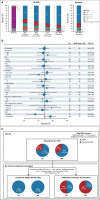
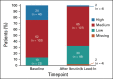
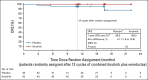
Results: One hundred sixty-four patients initiated three cycles of ibrutinib lead-in. After 12 cycles of ibrutinib plus venetoclax, best uMRD response rates were 75% (peripheral blood) and 68% (bone marrow). Patients with Confirmed uMRD were randomly assigned to receive placebo (n = 43) or ibrutinib (n = 43); patients with uMRD Not Confirmed were randomly assigned to ibrutinib (n = 31) or ibrutinib plus venetoclax (n = 32). Median follow-up was 31.3 months. One-year DFS rate was not significantly different between placebo (95%) and ibrutinib (100%; arm difference: 4.7% [95% CI, -1.6 to 10.9]; P = .15) in the Confirmed uMRD population. After ibrutinib lead-in tumor debulking, 36 of 40 patients (90%) with high tumor lysis syndrome risk at baseline shifted to medium or low tumor lysis syndrome risk categories. Adverse events were most frequent during the first 6 months of ibrutinib plus venetoclax and generally decreased over time.
Conclusion: The 1-year DFS rate of 95% in placebo-randomly assigned patients with Confirmed uMRD suggests the potential for fixed-duration treatment with this all-oral, once-daily, chemotherapy-free regimen in first-line CLL.
Conflict of interest statement
Figures

References
-
- Burger JA: Treatment of chronic lymphocytic leukemia. N Engl J Med 383:460-473, 2020 - PubMed
-
- VENCLEXTA (Venetoclax Tablets) for Oral Use [Package Insert]. South San Francisco, CA, Genentech USA, 2020
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

