Microglia regulate brain progranulin levels through the endocytosis/lysosomal pathway
- PMID: 34618685
- PMCID: PMC8663778
- DOI: 10.1172/jci.insight.136147
Microglia regulate brain progranulin levels through the endocytosis/lysosomal pathway
Abstract
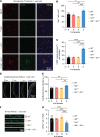
Genetic variants in Granulin (GRN), which encodes the secreted glycoprotein progranulin (PGRN), are associated with several neurodegenerative diseases, including frontotemporal lobar degeneration, neuronal ceroid lipofuscinosis, and Alzheimer's disease. These genetic alterations manifest in pathological changes due to a reduction of PGRN expression; therefore, identifying factors that can modulate PGRN levels in vivo would enhance our understanding of PGRN in neurodegeneration and could reveal novel potential therapeutic targets. Here, we report that modulation of the endocytosis/lysosomal pathway via reduction of Nemo-like kinase (Nlk) in microglia, but not in neurons, can alter total brain Pgrn levels in mice. We demonstrate that Nlk reduction promotes Pgrn degradation by enhancing its trafficking through the endocytosis/lysosomal pathway, specifically in microglia. Furthermore, genetic interaction studies in mice showed that Nlk heterozygosity in Grn haploinsufficient mice further reduces Pgrn levels and induces neuropathological phenotypes associated with PGRN deficiency. Our results reveal a mechanism for Pgrn level regulation in the brain through the active catabolism by microglia and provide insights into the pathophysiology of PGRN-associated diseases.
Keywords: Dementia; Mouse models; Neurodegeneration; Neuroscience.
Figures
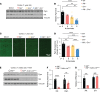
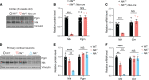
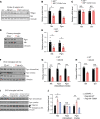
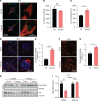

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

