Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study
- PMID: 34619098
- PMCID: PMC8489881
- DOI: 10.1016/S0140-6736(21)02183-8
Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study
Abstract
Background: Vaccine effectiveness studies have not differentiated the effect of the delta (B.1.617.2) variant and potential waning immunity in observed reductions in effectiveness against SARS-CoV-2 infections. We aimed to evaluate overall and variant-specific effectiveness of BNT162b2 (tozinameran, Pfizer-BioNTech) against SARS-CoV-2 infections and COVID-19-related hospital admissions by time since vaccination among members of a large US health-care system.
Methods: In this retrospective cohort study, we analysed electronic health records of individuals (≥12 years) who were members of the health-care organisation Kaiser Permanente Southern California (CA, USA), to assess BNT162b2 vaccine effectiveness against SARS-CoV-2 infections and COVID-19-related hospital admissions for up to 6 months. Participants were required to have 1 year or more previous membership of the organisation. Outcomes comprised SARS-CoV-2 PCR-positive tests and COVID-19-related hospital admissions. Effectiveness calculations were based on hazard ratios from adjusted Cox models. This study was registered with ClinicalTrials.gov, NCT04848584.
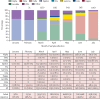
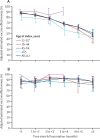
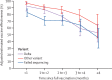
Findings: Between Dec 14, 2020, and Aug 8, 2021, of 4 920 549 individuals assessed for eligibility, we included 3 436 957 (median age 45 years [IQR 29-61]; 1 799 395 [52·4%] female and 1 637 394 [47·6%] male). For fully vaccinated individuals, effectiveness against SARS-CoV-2 infections was 73% (95% CI 72-74) and against COVID-19-related hospital admissions was 90% (89-92). Effectiveness against infections declined from 88% (95% CI 86-89) during the first month after full vaccination to 47% (43-51) after 5 months. Among sequenced infections, vaccine effectiveness against infections of the delta variant was high during the first month after full vaccination (93% [95% CI 85-97]) but declined to 53% [39-65] after 4 months. Effectiveness against other (non-delta) variants the first month after full vaccination was also high at 97% (95% CI 95-99), but waned to 67% (45-80) at 4-5 months. Vaccine effectiveness against hospital admissions for infections with the delta variant for all ages was high overall (93% [95% CI 84-96]) up to 6 months.
Interpretation: Our results provide support for high effectiveness of BNT162b2 against hospital admissions up until around 6 months after being fully vaccinated, even in the face of widespread dissemination of the delta variant. Reduction in vaccine effectiveness against SARS-CoV-2 infections over time is probably primarily due to waning immunity with time rather than the delta variant escaping vaccine protection.
Funding: Pfizer.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests JMZ, SG, KP, FJA, LJ, SRV, and JMM are employees of and hold stock and stock options in Pfizer. TBF holds shares of Pfizer stock. SYT, JMS, HF, VH, BKA, ONR, TBF, and OAO received research support from Pfizer during the conduct of this study that was paid directly to KPSC. For work unrelated to this project, SYT received research funding from Gilead, GlaxoSmithKline, and Genentech; BKA received research funding from GlaxoSmithKline, Novavax, Dynavax, Genentech, Novartis, Seqirus, and Moderna; JMS received research funding from Novavax, Dynavax, and ALK; and HF received research funding from Genentech. All other authors declare no competing interests.
Figures
Comment in
-
Developing COVID-19 vaccine policy in increments.Lancet. 2021 Oct 16;398(10309):1382-1383. doi: 10.1016/S0140-6736(21)02242-X. Lancet. 2021. PMID: 34656213 Free PMC article. No abstract available.
References
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. - PMC - PubMed
-
- Haas EJ, McLaughlin JM, Khan F, et al. Infections, hospitalizations, and deaths averted via direct effects of the Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccine in a nationwide vaccination campaign, Israel. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00566-1. published online Sept 22. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

