Third-party type 2 innate lymphoid cells prevent and treat GI tract GvHD
- PMID: 34619767
- PMCID: PMC8759141
- DOI: 10.1182/bloodadvances.2020001514
Third-party type 2 innate lymphoid cells prevent and treat GI tract GvHD
Abstract
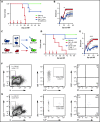
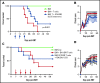
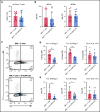
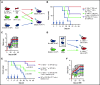
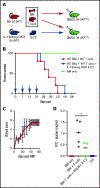
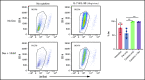
Acute graft-versus-host disease (aGVHD), mediated by the recognition of host major histocompatibility complex/peptide polymorphisms by donor T cells, remains a significant complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT). aGVHD most commonly involves the gastrointestinal tract, liver, and skin; symptomatic aGVHD is treated with corticosteroids. Steroid-nonresponsive aGVHD is a significant problem for patients undergoing allo-HSCT, with <15% of these patients alive 1 year after diagnosis. Previously, we found that the infusion of donor innate lymphoid type 2 (ILC2) cells could prevent and treat aGVHD of the lower gastrointestinal tract with no effect on the graft-versus-leukemia response. This approach for clinical translation is cumbersome, as it would require the generation of donor-derived ILC2 cells for each recipient. Thus, the ability to use third-party ILC2 cells would provide an "off-the-shelf" reagent that could be used to treat and/or prevent aGVHD. Here, we show that third-party ILC2 cells enhance the survival of allo-HSCT recipients. Treatment required at least 4 weekly infusions of ILC2 cells. Mechanistically, we show that ILC2 cell function was completely lost if the cells could not express both interleukin-13 (IL-13) and amphiregulin. Finally, we show that the activity of IL-13 has a greater dependence on the expression of the IL-13R on host rather than donor bone marrow cells. The ability to generate third-party ILC2 cells offers a new avenue for the prevention of aGVHD.
© 2021 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures


References
-
- Horwitz ME. Reduced intensity versus myeloablative allogeneic stem cell transplantation for the treatment of acute myeloid leukemia, myelodysplastic syndrome and acute lymphoid leukemia. Curr Opin Oncol. 2011;23(2):197-202. - PubMed
-
- Kersey JH. The role of allogeneic-cell transplantation in leukemia. N Engl J Med. 2010;363(22):2158-2159. - PubMed
-
- Gatti RA, Kersey JH, Yunis EJ, Good RA.. Graft-versus-host disease. Prog Clin Pathol. 1973;5:1-18. - PubMed

