A scintigraphy study of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler in patients with moderate-to-very severe chronic obstructive pulmonary disease
- PMID: 34620167
- PMCID: PMC8496011
- DOI: 10.1186/s12931-021-01813-w
A scintigraphy study of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler in patients with moderate-to-very severe chronic obstructive pulmonary disease
Abstract
Background: Triple therapy with inhaled corticosteroids/long-acting muscarinic antagonists/long-acting β2-agonists (ICS/LAMA/LABA) is recommended for patients with chronic obstructive pulmonary disease (COPD) with continued symptoms or exacerbations, despite treatment with LAMA/LABA or ICS/LABA. The pulmonary, extrathoracic, and regional lung deposition patterns of a radiolabeled ICS/LAMA/LABA triple fixed-dose combination budesonide/glycopyrrolate/formoterol fumarate (BGF 320/18/9.6 μg), delivered via a single Aerosphere metered dose inhaler (MDI) were previously assessed in healthy volunteers and showed good deposition to the central and peripheral airways (whole lung deposition: 37.7%). Here, we report the findings assessing BGF in patients with moderate-to-very severe COPD.
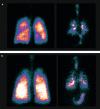
Methods: This phase I, single-dose, open-label gamma scintigraphy imaging study (NCT03906045) was conducted in patients with moderate-to-very severe COPD. Patients received two actuations of BGF MDI (160/9/4.8 μg per actuation) radiolabeled with technetium‑99‑pertechnetate, not exceeding 5 MBq per actuation. Immediately following each inhalation, patients performed a breath-hold of up to 10 s, then exhaled into an exhalation filter. Gamma scintigraphy imaging of the anterior and posterior views of the lungs and stomach, and a lateral head and neck view, were performed immediately after exhalation. The primary objective of the study was to assess the pulmonary deposition of BGF. Secondary objectives assessed the deposited dose of radiolabeled BGF in the oropharyngeal and stomach regions, on the actuator, and on the exhalation filter in addition to regional airway deposition patterns in the lungs.
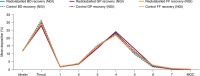
Results: The mean BGF emitted dose deposited in the lungs was 32.1% (standard deviation [SD] 15.6) in patients with moderate-to-very severe COPD, 35.2% (SD 12.8) in patients with moderate COPD, and 28.7% (SD 18.4) in patients with severe/very severe COPD. Overall, the mean normalized outer/inner ratio was 0.55 (SD 0.19), while the standardized central/peripheral ratio was 2.21 (SD 1.64).
Conclusions: Radiolabeled BGF 320/18/9.6 μg was efficiently delivered and deposited throughout the entire lung, including large and small airways, in patients with moderate-to-very severe COPD, with similar deposition in patients with moderate COPD and patients with severe/very severe COPD.
Trial registration: ClinicalTrials.gov, NCT03906045. Registered 8 April 2019, https://clinicaltrials.gov/ct2/show/NCT03906045.
Keywords: Budesonide; Formoterol fumarate; Gamma scintigraphy; Glycopyrronium; Pulmonary deposition.
© 2021. The Author(s).
Conflict of interest statement
OU reports academic grants from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline; and personal fees from Aerocrine, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Mundipharma, NAPP, Prosonix Ltd, Sandoz, Takeda, and Zentiva NR reports grants and personal fees from Boehringer Ingelheim, Novartis, and Pfizer; and personal fees from AstraZeneca, Chiesi, Cipla, GlaxoSmithKline, Mundipharma, Sanofi, Teva, Trudell, and Zambon. EW and SI declare that they have no competing interests. MJ, RT, PD, and MA are employees of, and hold stock and/or stock options in, AstraZeneca.
Figures


References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2021 report. 2021. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25.... Accessed 5 Apr 2021. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

