Therapeutic effects of mesenchymal stem cells-derived extracellular vesicles' miRNAs on retinal regeneration: a review
- PMID: 34620234
- PMCID: PMC8499475
- DOI: 10.1186/s13287-021-02588-z
Therapeutic effects of mesenchymal stem cells-derived extracellular vesicles' miRNAs on retinal regeneration: a review
Abstract
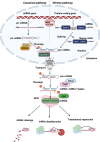
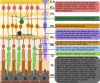
Extracellular vesicles (EVs), which consist of microvesicles and exosomes, are secreted from all cells to transform vital information in the form of lipids, proteins, mRNAs and small RNAs such as microRNAs (miRNAs). Many studies demonstrated that EVs' miRNAs have effects on target cells. Numerous people suffer from the blindness caused by retinal degenerations. The death of retinal neurons is irreversible and creates permanent damage to the retina. In the absence of acceptable cures for retinal degenerative diseases, stem cells and their paracrine agents including EVs have become a promising therapeutic approach. Several studies showed that the therapeutic effects of stem cells are due to the miRNAs of their EVs. Considering the effects of microRNAs in retinal cells development and function and studies which provide the possible roles of mesenchymal stem cells-derived EVs miRNA content on retinal diseases, we focused on the similarities between these two groups of miRNAs that could be helpful for promoting new therapeutic techniques for retinal degenerative diseases.
Keywords: Extracellular vesicles; Mesenchymal stem cells; Retina; miRNA.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

