Localizing the Epileptogenic Zone with Novel Biomarkers
- PMID: 34620466
- PMCID: PMC8501232
- DOI: 10.1016/j.spen.2021.100919
Localizing the Epileptogenic Zone with Novel Biomarkers
Abstract
Several noninvasive methods, such as high-density EEG or magnetoencephalography, are currently used to delineate the epileptogenic zone (EZ) during the presurgical evaluation of patients with drug resistant epilepsy (DRE). Yet, none of these methods can reliably identify the EZ by their own. In most cases a multimodal approach is needed. Challenging cases often require the implantation of intracranial electrodes, either through stereo-taxic EEG or electro-corticography. Recently, a growing body of literature introduces novel biomarkers of epilepsy that can be used for analyzing both invasive as well as noninvasive electrophysiological data. Some of these biomarkers are able to delineate the EZ with high precision, augment the presurgical evaluation, and predict the surgical outcome of patients with DRE undergoing surgery. However, the use of these epilepsy biomarkers in clinical practice is limited. Here, we summarize and discuss the latest technological advances in the presurgical neurophysiological evaluation of children with DRE with emphasis on electric and magnetic source imaging, high frequency oscillations, and functional connectivity.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

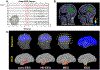
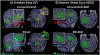


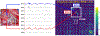


References
-
- Ryvlin P, Cross JH, Rheims S. Epilepsy surgery in children and adults. Lancet Neurol 2014; 13(11): 1114–26. - PubMed
-
- Dwivedi R, Ramanujam B, Chandra PS, Sapra S, Gulati S, Kalaivani M, Garg A, Bal CS, Tripathi M, Dwivedi SN, Sagar R, Sarkar C, Tripathi M. Surgery for Drug-Resistant Epilepsy in Children. N Engl J Med. 2017; 377(17): 1639–1647. - PubMed
-
- Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001; 124(Pt 9): 1683–700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

