A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: An interim analysis in Indonesia
- PMID: 34620531
- PMCID: PMC8461222
- DOI: 10.1016/j.vaccine.2021.09.052
A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: An interim analysis in Indonesia
Abstract
Background: The WHO declared COVID-19 a pandemic on March 11th, 2020. This serious outbreak and the precipitously increasing numbers of deaths worldwide necessitated the urgent need to develop an effective severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine. The development of COVID-19 vaccines has moved quickly. In this study, we assessed the efficacy, safety, and immunogenicity of an inactivated (SARS-CoV-2) vaccine.
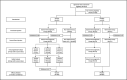
Methods: We conducted a randomized, double-blind, placebo-controlled trial to evaluate the efficacy, immunogenicity, and safety of an inactivated SARS-CoV-2 vaccine and its lot-to-lot consistency. A total of 1620 healthy adults aged 18-59 years were randomly assigned to receive 2 injections of the trial vaccine or placebo on a day 0 and 14 schedule. This article was based on an interim report completed within 3 months following the last dose of study vaccine. The interim analysis includes safety and immunogenicity data for 540 participants in the immunogenicity subset and an efficacy analysis of the 1620 subjects. For the safety evaluation, solicited and unsolicited adverse events were collected after the first and second vaccination within 14 and 28 days, respectively. Blood samples were collected for an antibody assay before and 14 days following the second dose.
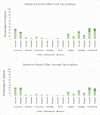
Results: Most of the adverse reactions were in the solicited category and were mild in severity. Pain at the injection site was the most frequently reported symptom. Antibody IgG titer determined by enzyme-linked immunosorbent assay was 97.48% for the seroconversion rate. Using a neutralization assay, the seroconversion rate was 87.15%. The efficacy in preventing symptomatic confirmed cases of COVID-19 occurring at least 14 days after the second dose of vaccine using an incidence rate was 65.30%.
Conclusions: From the 3-month interim analysis, the vaccine exhibited a 65.30% efficacy at preventing COVID-19 illness with favorable safety and immunogenicity profiles.
Keywords: Adults; Efficacy; Immunogenicity; SARS-CoV-2 Inactivated Vaccine; Safety.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Ophinni Y., Hasibuan A.S., Widhani A., Maria S., Koesnoe S., Yunihastuti E., et al. COVID-19 Vaccines: Current Status and Implication for Use in Indonesia. Acta Med Indones. 2020;52:388–412. - PubMed
-
- WHO Coronavirus disease (COVID-19) Situation Report-132. 2020
-
- Gupta S.D. Coronavirus Pandemic: A Serious Threat to Humanity. J Health Manag. 2020;22(1):1–2. doi: 10.1177/0972063420921260. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous