18F-FDG PET/CT for Posttreatment Surveillance Imaging of Patients with Stage III Merkel Cell Carcinoma
- PMID: 34620729
- PMCID: PMC9157731
- DOI: 10.2967/jnumed.121.262882
18F-FDG PET/CT for Posttreatment Surveillance Imaging of Patients with Stage III Merkel Cell Carcinoma
Abstract
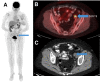
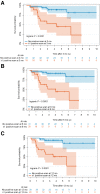
The purpose of this study was to investigate the diagnostic and prognostic value of 18F-FDG PET/CT for surveillance imaging in patients treated for stage III Merkel cell carcinoma (MCC). Methods: This retrospective study included 61 consecutive stage III MCC patients who were clinically asymptomatic and underwent surveillance 18F-FDG PET/CT. Findings were correlated with either pathology or clinical/imaging follow-up. The median follow-up period was 4.8 y. Statistical analyses were performed. Results:18F-FDG PET/CT detected unsuspected recurrences in 33% patients (20/61) with lesion-based sensitivity, specificity, and accuracy of 92%, 93%, and 93%, respectively. The mean ± SD SUV for malignant and benign lesions was 7.5 ± 3.9 and 3.8 ± 2.0, respectively. Unknown distant metastases, as first recurrence site, were noted in 12 of 61 patients. Those with positive disease on 18F-FDG PET/CT within 1 y of definitive treatment had relatively worse overall survival (P < 0.0001). After adjustment on stage, risk of death increased with a higher SUVmax (hazard ratio for 1 unit = 1.17; P = 0.006) and with a higher number of positive lesions on 18F-FDG PET/CT (hazard ratio for 1 additional lesion = 1.60; P < 0.001). Conclusion: Postdefinitive treatment surveillance 18F-FDG PET/CT scanning detects unsuspected recurrences and has prognostic value. Inclusion of 18F-FDG PET/CT within the first 6 mo after definitive treatment would be appropriate for surveillance and early detection of recurrence. Our data merit further studies to evaluate the prognostic implications.
Keywords: 18F-FDG PET/CT; Merkel cell carcinoma; PET; prognosis; recurrence; surveillance.
© 2022 by the Society of Nuclear Medicine and Molecular Imaging.
Figures


References
-
- Becker JC. Merkel cell carcinoma. Ann Oncol. 2010;21:vii81–vii85. - PubMed
-
- Guidelines NCCN. Merkel cell carcinoma. Version 1.2020. Available from NCCNorg website. https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf. Accessed March 7, 2022.
-
- Fields RC, Busam KJ, Chou JF, et al. . Five hundred patients with Merkel cell carcinoma evaluated at a single institution. Ann Surg. 2011;254:465–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
