Next generation of tumor-activating type I IFN enhances anti-tumor immune responses to overcome therapy resistance
- PMID: 34620867
- PMCID: PMC8497482
- DOI: 10.1038/s41467-021-26112-2
Next generation of tumor-activating type I IFN enhances anti-tumor immune responses to overcome therapy resistance
Abstract
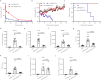
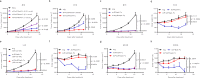
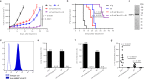
Type I interferon is promising in treating different kinds of tumors, but has been limited by its toxicity, lack of tumor targeting, and very short half-life. To target tumors, reduce systemic toxicity, and increase half-life, here we engineer a masked type I IFN-Fc (ProIFN) with its natural receptor connected by a cleavable linker that can be targeted by tumor-associated proteases. ProIFN has a prolonged serum half-life and shows an improved tumor-targeting effect. Interestingly, ProIFN-treated mice show enhanced DC cross-priming and significant increased CD8+ infiltration and effector function in the tumor microenvironment. ProIFN is able to improve checkpoint blockade efficacy in established tumors, as well as radiation efficacy for both primary and metastatic tumors. ProIFN exhibits superior long-term pharmacokinetics with minimal toxicity in monkeys. Therefore, this study demonstrates an effective tumor-activating IFN that can increase targeted immunity against primary tumor or metastasis and reduce periphery toxicity to the host.
© 2021. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: Dr. Yang-Xin Fu and Xuezhi Cao are co-inventors on a US provisional patent “Interferon prodrug for the treatment of cancer”, application Ser. No. 62/522,564, which incorporates discoveries described in this manuscript. The patent application was filed by The Board Of Regents Of The University Of Texas System. Zhenxiang Hu and Jiaming Yang are the employees of Livzon. Other authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

