Contamination-resistant, rapid emulsion-based isothermal nucleic acid amplification with Mie-scatter inspired light scatter analysis for bacterial identification
- PMID: 34620908
- PMCID: PMC8497611
- DOI: 10.1038/s41598-021-99200-4
Contamination-resistant, rapid emulsion-based isothermal nucleic acid amplification with Mie-scatter inspired light scatter analysis for bacterial identification
Abstract
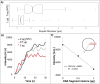
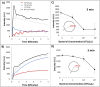
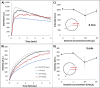
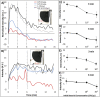
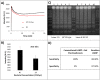
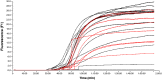
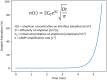
An emulsion loop-mediated isothermal amplification (eLAMP) platform was developed to reduce the impact that contamination has on assay performance. Ongoing LAMP reactions within the emulsion droplets cause a decrease in interfacial tension, causing a decrease in droplet size, which results in decreased light scatter intensity due to Mie theory. Light scatter intensity was monitored via spectrophotometers and fiber optic cables placed at 30° and 60°. Light scatter intensities collected at 3 min, 30° were able to statistically differentiate 103 and 106 CFU/µL initial Escherichia coli O157:H7 concentrations compared to NTC (0 CFU/µL), while the intensity at 60° were able to statistically differentiate 106 CFU/µL initial concentrations and NTC. Control experiments were conducted to validate nucleic acid detection versus bacterial adsorption, finding that the light scatter intensities change is due specifically to ongoing LAMP amplification. After inducing contamination of bulk LAMP reagents, specificity lowered to 0% with conventional LAMP, while the eLAMP platform showed 87.5% specificity. We have demonstrated the use of angle-dependent light scatter intensity as a means of real-time monitoring of an emulsion LAMP platform and fabricated a smartphone-based monitoring system that showed similar trends as spectrophotometer light scatter data, validating the technology for a field deployable platform.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Emulsion-based isothermal nucleic acid amplification for rapid SARS-CoV-2 detection via angle-dependent light scatter analysis.Biosens Bioelectron. 2021 May 1;179:113099. doi: 10.1016/j.bios.2021.113099. Epub 2021 Feb 19. Biosens Bioelectron. 2021. PMID: 33640656 Free PMC article.
-
Mie Scatter and Interfacial Tension Based Real-Time Quantification of Colloidal Emulsion Nucleic Acid Amplification.Adv Biosyst. 2017 Oct;1(10):e1700098. doi: 10.1002/adbi.201700098. Epub 2017 Sep 7. Adv Biosyst. 2017. PMID: 32646190
-
Interfacial Effect-Based Quantification of Droplet Isothermal Nucleic Acid Amplification for Bacterial Infection.Sci Rep. 2019 Jul 3;9(1):9629. doi: 10.1038/s41598-019-46028-8. Sci Rep. 2019. PMID: 31270374 Free PMC article.
-
Loop-mediated isothermal amplification assay as a point-of-care diagnostic tool for Vibrio parahaemolyticus: recent developments and improvements.Expert Rev Mol Diagn. 2019 Mar;19(3):229-239. doi: 10.1080/14737159.2019.1571913. Epub 2019 Jan 28. Expert Rev Mol Diagn. 2019. PMID: 30657706 Review.
-
Loop-mediated isothermal amplification (LAMP): A novel rapid detection platform for pathogens.Microb Pathog. 2017 Jun;107:54-61. doi: 10.1016/j.micpath.2017.03.016. Epub 2017 Mar 18. Microb Pathog. 2017. PMID: 28323152 Review.
Cited by
-
Progression of LAMP as a Result of the COVID-19 Pandemic: Is PCR Finally Rivaled?Biosensors (Basel). 2022 Jul 6;12(7):492. doi: 10.3390/bios12070492. Biosensors (Basel). 2022. PMID: 35884295 Free PMC article. Review.
-
Machine learning classification of bacterial species using mix-and-match reagents on paper microfluidic chips and smartphone-based capillary flow analysis.Anal Bioanal Chem. 2022 May;414(13):3895-3904. doi: 10.1007/s00216-022-04031-5. Epub 2022 Mar 28. Anal Bioanal Chem. 2022. PMID: 35347355
References
-
- ThermoFisher. PCR Basics, ThermoFisher Scientific: Waltham. https://www.thermofisher.com/us/en/home/life-science/cloning/cloning-lea... (accessed November 12, 2019).
-
- Sigma-Aldrich. SYBR Green Based Quantitative PCR, Sigma-Aldrich: St. Louis. https://www.sigmaaldrich.com/life-science/molecular-biology/pcr/quantita... (accessed November 11, 2019).
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

