Functional characterization of a bioengineered liver after heterotopic implantation in pigs
- PMID: 34620986
- PMCID: PMC8497596
- DOI: 10.1038/s42003-021-02665-2
Functional characterization of a bioengineered liver after heterotopic implantation in pigs
Erratum in
-
Author Correction: Functional characterization of a bioengineered liver after heterotopic implantation in pigs.Commun Biol. 2021 Dec 8;4(1):1393. doi: 10.1038/s42003-021-02929-x. Commun Biol. 2021. PMID: 34880384 Free PMC article. No abstract available.
Abstract
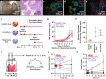
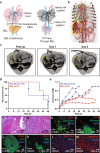
Organ bioengineering offers a promising solution to the persistent shortage of donor organs. However, the progression of this technology toward clinical use has been hindered by the challenges of reconstituting a functional vascular network, directing the engraftment of specific functional cell types, and defining appropriate culture conditions to concurrently support the health and phenotypic stability of diverse cell lineages. We previously demonstrated the ability to functionally reendothelialize the vasculature of a clinically scaled decellularized liver scaffold with human umbilical vein endothelial cells (HUVECs) and to sustain continuous perfusion in a large animal recovery model. We now report a method for seeding and engrafting primary porcine hepatocytes into a bioengineered liver (BEL) scaffold previously reendothelialized with HUVECs. The resulting BELs were competent for albumin production, ammonia detoxification and urea synthesis, indicating the presence of a functional hepatocyte compartment. BELs additionally slowed ammonia accumulation during in vivo perfusion in a porcine model of surgically induced acute liver failure. Following explant of the graft, BEL parenchyma showed maintenance of canonical endothelial and hepatocyte markers. Taken together, these results support the feasibility of engineering a clinically scaled functional BEL and establish a platform for optimizing the seeding and engraftment of additional liver specific cells.
© 2021. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: B.D.A., A.A.K., A.M., B.G.S., A.R.S., V.L.N., R.N.P., T.W.G., D.S.D., and J.J.R. are employees of Miromatrix Inc. Miromatrix Inc. is a publicly funded company and owns the exclusive patent rights for the perfusion decellularization and recellularization technologies utilized in this study. The remaining authors declare no competing interests.
Figures


Comment in
-
Bioengineered Organs: Not a Matter of "If".Am J Transplant. 2022 Jan;22(1):1-2. doi: 10.1111/ajt.16639. Am J Transplant. 2022. PMID: 34967122 No abstract available.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

