Safety, pharmacokinetics and antiviral activity of PGT121, a broadly neutralizing monoclonal antibody against HIV-1: a randomized, placebo-controlled, phase 1 clinical trial
- PMID: 34621054
- PMCID: PMC8516645
- DOI: 10.1038/s41591-021-01509-0
Safety, pharmacokinetics and antiviral activity of PGT121, a broadly neutralizing monoclonal antibody against HIV-1: a randomized, placebo-controlled, phase 1 clinical trial
Abstract
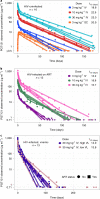
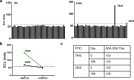
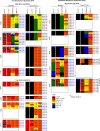
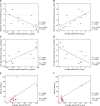
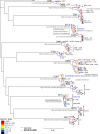
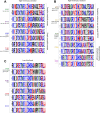
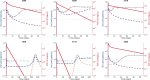
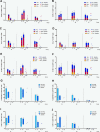
Human immunodeficiency virus (HIV)-1-specific broadly neutralizing monoclonal antibodies are currently under development to treat and prevent HIV-1 infection. We performed a single-center, randomized, double-blind, dose-escalation, placebo-controlled trial of a single administration of the HIV-1 V3-glycan-specific antibody PGT121 at 3, 10 and 30 mg kg-1 in HIV-uninfected adults and HIV-infected adults on antiretroviral therapy (ART), as well as a multicenter, open-label trial of one infusion of PGT121 at 30 mg kg-1 in viremic HIV-infected adults not on ART (no. NCT02960581). The primary endpoints were safety and tolerability, pharmacokinetics (PK) and antiviral activity in viremic HIV-infected adults not on ART. The secondary endpoints were changes in anti-PGT121 antibody titers and CD4+ T-cell count, and development of HIV-1 sequence variations associated with PGT121 resistance. Among 48 participants enrolled, no treatment-related serious adverse events, potential immune-mediated diseases or Grade 3 or higher adverse events were reported. The most common reactions among PGT121 recipients were intravenous/injection site tenderness, pain and headache. Absolute and relative CD4+ T-cell counts did not change following PGT121 infusion in HIV-infected participants. Neutralizing anti-drug antibodies were not elicited. PGT121 reduced plasma HIV RNA levels by a median of 1.77 log in viremic participants, with a viral load nadir at a median of 8.5 days. Two individuals with low baseline viral loads experienced ART-free viral suppression for ≥168 days following antibody infusion, and rebound viruses in these individuals demonstrated full or partial PGT121 sensitivity. The trial met the prespecified endpoints. These data suggest that further investigation of the potential of antibody-based therapeutic strategies for long-term suppression of HIV is warranted, including in individuals off ART and with low viral load.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 OD011095/OD/NIH HHS/United States
- U01 AI145801/AI/NIAID NIH HHS/United States
- P01 AI131365/AI/NIAID NIH HHS/United States
- U01 CA260476/CA/NCI NIH HHS/United States
- R01 AI028433/AI/NIAID NIH HHS/United States
- R01 OD024917/OD/NIH HHS/United States
- UL1 TR001102/TR/NCATS NIH HHS/United States
- UM1 AI124377/AI/NIAID NIH HHS/United States
- U19 AI128751/AI/NIAID NIH HHS/United States
- K08 AI106408/AI/NIAID NIH HHS/United States
- K23 AI114381/AI/NIAID NIH HHS/United States
- R01 AI129797/AI/NIAID NIH HHS/United States
- R01 AI149670/AI/NIAID NIH HHS/United States
- UM1 AI126603/AI/NIAID NIH HHS/United States
- R37 AI028433/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

