Drug Repurposing Prediction and Validation From Clinical Big Data for the Effective Treatment of Interstitial Lung Disease
- PMID: 34621164
- PMCID: PMC8490809
- DOI: 10.3389/fphar.2021.635293
Drug Repurposing Prediction and Validation From Clinical Big Data for the Effective Treatment of Interstitial Lung Disease
Abstract
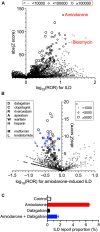
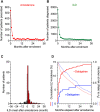
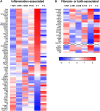
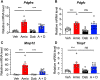
Interstitial lung diseases (ILDs) are a group of respiratory disorders characterized by chronic inflammation and fibrosis of the pulmonary interstitial tissues. Although the etiology of ILD remains unclear, some drug treatments are among the primary causes of ILD. In the present study, we analyzed the FDA Adverse Event Reporting System and JMDC Inc. insurance claims to identify a coexisting drug that reduced the incidence of ILD associated with the use of an anti-arrhythmic agent, amiodarone, and found that the thrombin inhibitor dabigatran prevented the amiodarone-induced ILD in both clinical datasets. In an experimental validation of the hypothesis, long-term oral treatment of mice with amiodarone caused a gradual decrease in body weight caused by respiratory insufficiency. In the lungs of amiodarone-treated mice, infiltration of macrophages was observed in parallel with a delayed upregulation of the platelet-derived growth factor receptor α gene. In contrast, co-treatment with dabigatran significantly attenuated these amiodarone-induced changes indicative of ILD. These results suggest that dabigatran is effective in preventing drug-induced ILD. This combinatorial approach of drug repurposing based on clinical big data will pave the way for finding a new treatment with high clinical predictability and a well-defined molecular mechanism.
Keywords: adverse event; amiodarone; chronic inflammation; dabigatran; pulmonary fibrosis; real-world data; retrospective analysis.
Copyright © 2021 Siswanto, Yamamoto, Furuta, Kobayashi, Nagashima, Kayanuma, Nagayasu, Imai and Kaneko.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bogatkevich G. S., Ludwicka-Bradley A., Nietert P. J., Akter T., van Ryn J., Silver R. M. (2011). Antiinflammatory and Antifibrotic Effects of the Oral Direct Thrombin Inhibitor Dabigatran Etexilate in a Murine Model of Interstitial Lung Disease. Arthritis Rheum. 63, 1416–1425. 10.1002/art.30255 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

