Volumetric MRI-Based Biomarkers in Huntington's Disease: An Evidentiary Review
- PMID: 34621236
- PMCID: PMC8490802
- DOI: 10.3389/fneur.2021.712555
Volumetric MRI-Based Biomarkers in Huntington's Disease: An Evidentiary Review
Abstract
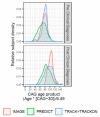
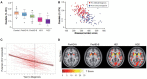
Huntington's disease (HD) is an autosomal-dominant inherited neurodegenerative disorder that is caused by expansion of a CAG-repeat tract in the huntingtin gene and characterized by motor impairment, cognitive decline, and neuropsychiatric disturbances. Neuropathological studies show that disease progression follows a characteristic pattern of brain atrophy, beginning in the basal ganglia structures. The HD Regulatory Science Consortium (HD-RSC) brings together diverse stakeholders in the HD community-biopharmaceutical industry, academia, nonprofit, and patient advocacy organizations-to define and address regulatory needs to accelerate HD therapeutic development. Here, the Biomarker Working Group of the HD-RSC summarizes the cross-sectional evidence indicating that regional brain volumes, as measured by volumetric magnetic resonance imaging, are reduced in HD and are correlated with disease characteristics. We also evaluate the relationship between imaging measures and clinical change, their longitudinal change characteristics, and within-individual longitudinal associations of imaging with disease progression. This analysis will be valuable in assessing pharmacodynamics in clinical trials and supporting clinical outcome assessments to evaluate treatment effects on neurodegeneration.
Keywords: C-Path; Huntington's disease; biomarkers; neurodegenerative; neuroimaging; volumetric MRI.
Copyright © 2021 Kinnunen, Schwarz, Turner, Pustina, Gantman, Gordon, Joules, Mullin, Scahill, Georgiou-Karistianis and The Huntington's Disease Regulatory Science Consortium (HD-RSC).
Conflict of interest statement
KMK and RJ are full-time employees of IXICO. AJS is a full-time employee and shareholder of Takeda Pharmaceuticals, Ltd. DP and ECG are employed by CHDI Management to provide advisory services to CHDI Foundation. MFG is a full-time employee of Teva Pharmaceuticals. APM is a full-time employee of Wave Life Sciences, Ltd. RIS provides consultancy for IXICO. The remaining authors declare that the research was conducted in the absence of any other commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, et al. . Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. (2009) 8:791–801. 10.1016/S1474-4422(09)70170-X - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

