Meta-Analysis of Interleukin-2 Receptor Antagonists as the Treatment for Steroid-Refractory Acute Graft- Versus-Host Disease
- PMID: 34621279
- PMCID: PMC8490710
- DOI: 10.3389/fimmu.2021.749266
Meta-Analysis of Interleukin-2 Receptor Antagonists as the Treatment for Steroid-Refractory Acute Graft- Versus-Host Disease
Abstract
Acute graft-versus-host disease (aGVHD) is a major complication after allogeneic hematopoietic stem cell transplantation (HSCT). Corticosteroid is the first-line treatment for aGVHD, but its response rate is only approximately 50%. At present, no uniformly accepted treatment for steroid-refractory aGVHD (SR-aGVHD) is available. Blocking interleukin-2 receptors (IL-2Rs) on donor T cells using pharmaceutical antagonists alleviates SR-aGVHD. This meta-analysis aimed to compare the efficacy and safety of four commercially available IL-2R antagonists (IL-2RAs) in SR-aGVHD treatment. A total of 31 studies met the following inclusion criteria (1): patients of any race, any sex, and all ages (2); those diagnosed with SR-aGVHD after HSCT; and (3) those using IL-2RA-based therapy as the treatment for SR-aGVHD. The overall response rate (ORR) at any time after treatment with basiliximab and daclizumab was 0.81 [95% confidence interval (CI): 0.74-0.87)] and 0.71 (95% CI: 0.56-0.82), respectively, which was better than that of inolimomab 0.54 (95% CI: 0.39-0.68) and denileukin diftitox 0.56 (95% CI: 0.35-0.76). The complete response rate (CRR) at any time after treatment with basiliximab and daclizumab was 0.55 (95% CI: 0.42-0.68) and 0.42 (95%CI: 0.29-0.56), respectively, which was better than that of inolimomab 0.30 (95% CI: 0.16-0.51) and denileukin diftitox 0.37 (95% CI: 0.24-0.52). The ORR and CRR were better after 1-month treatment with basiliximab and daclizumab than after treatment with inolimomab and denileukin diftitox. The incidence of the infection was higher after inolimomab treatment than after treatment with the other IL-2RAs. In conclusion, the efficacy and safety of different IL-2RAs varied. The response rate of basiliximab was the highest, followed by that of daclizumab. Prospective, randomized controlled trials are needed to compare the efficacy and safety of different IL-2RAs.
Keywords: acute graft-versus-host disease; interleukin-2 receptor antagonist; meta-analysis; second-line treatment; steroid-refractory.
Copyright © 2021 Shen, Li, Zhang, Xu, Wang, Liu, Huang, Hong and Mo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
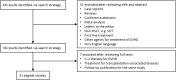


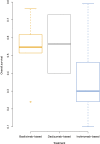
Similar articles
-
Basiliximab Treatment for Patients With Steroid-Refractory Acute Graft-Versus-Host Disease Following Matched Sibling Donor Hematopoietic Stem Cell Transplantation.Cell Transplant. 2024 Jan-Dec;33:9636897241257568. doi: 10.1177/09636897241257568. Cell Transplant. 2024. PMID: 38832653 Free PMC article.
-
Second-line therapy for patients with steroid-refractory aGVHD: systematic review and meta-analysis of randomized controlled trials.Front Immunol. 2023 Jun 20;14:1211171. doi: 10.3389/fimmu.2023.1211171. eCollection 2023. Front Immunol. 2023. PMID: 37409129 Free PMC article.
-
Prophylactic effects of interleukin-2 receptor antagonists against graft-versus-host disease following unrelated donor peripheral blood stem cell transplantation.Biol Blood Marrow Transplant. 2012 May;18(5):754-62. doi: 10.1016/j.bbmt.2011.09.005. Epub 2011 Sep 29. Biol Blood Marrow Transplant. 2012. PMID: 21963619
-
Vedolizumab plus basiliximab as second-line therapy for steroid-refractory lower gastrointestinal acute graft-versus-host disease.Front Immunol. 2024 Jul 3;15:1408211. doi: 10.3389/fimmu.2024.1408211. eCollection 2024. Front Immunol. 2024. PMID: 39021571 Free PMC article.
-
Interleukin-2 receptor antagonists for pediatric liver transplant recipients: a systematic review and meta-analysis of controlled studies.Pediatr Transplant. 2014 Dec;18(8):839-50. doi: 10.1111/petr.12362. Epub 2014 Oct 4. Pediatr Transplant. 2014. PMID: 25283839
Cited by
-
Efficacy and safety of ruxolitinib in steroid-refractory graft-versus-host disease: A meta-analysis.Front Immunol. 2022 Aug 4;13:954268. doi: 10.3389/fimmu.2022.954268. eCollection 2022. Front Immunol. 2022. PMID: 35990629 Free PMC article.
-
Basiliximab Treatment for Patients With Steroid-Refractory Acute Graft-Versus-Host Disease Following Matched Sibling Donor Hematopoietic Stem Cell Transplantation.Cell Transplant. 2024 Jan-Dec;33:9636897241257568. doi: 10.1177/09636897241257568. Cell Transplant. 2024. PMID: 38832653 Free PMC article.
-
Immune Reconstitution of Patients Who Recovered From Steroid-Refractory Acute Graft-Versus-Host Disease After Basiliximab Treatment.Front Oncol. 2022 Jul 15;12:916442. doi: 10.3389/fonc.2022.916442. eCollection 2022. Front Oncol. 2022. PMID: 35936697 Free PMC article.
-
Second-line therapy for patients with steroid-refractory aGVHD: systematic review and meta-analysis of randomized controlled trials.Front Immunol. 2023 Jun 20;14:1211171. doi: 10.3389/fimmu.2023.1211171. eCollection 2023. Front Immunol. 2023. PMID: 37409129 Free PMC article.
-
Humanized anti-CD25 monoclonal antibody replaces methotrexate as acute graft-versus-host disease prophylaxis in haploidentical allogeneic haematopoietic stem cell transplantation.Br J Haematol. 2025 Feb;206(2):615-627. doi: 10.1111/bjh.19958. Epub 2024 Dec 22. Br J Haematol. 2025. PMID: 39710371 Free PMC article.
References
-
- Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N, et al. . Treatment of Moderate/Severe Acute Graft-Versus-Host Disease After Allogeneic Bone Marrow Transplantation: An Analysis of Clinical Risk Features and Outcome. Blood (1990) 75(4):1024–30. doi: 10.1182/blood.V75.4.1024.1024 - DOI - PubMed
-
- Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. . First- and Second-Line Systemic Treatment of Acute Graft-Versus-Host Disease: Recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant (2012) 18(8):1150–63. doi: 10.1016/j.bbmt.2012.04.005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

